A conserved epitope in VAR2CSA is targeted by a cross-reactive antibody originating from Plasmodium vivax Duffy binding protein
- PMID: 37396303
- PMCID: PMC10312377
- DOI: 10.3389/fcimb.2023.1202276
A conserved epitope in VAR2CSA is targeted by a cross-reactive antibody originating from Plasmodium vivax Duffy binding protein
Abstract
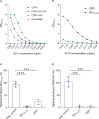
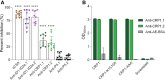
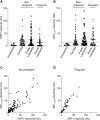
During Plasmodium falciparum infection in pregnancy, VAR2CSA is expressed on the surface of infected erythrocytes (IEs) and mediates their sequestration in the placenta. As a result, antibodies to VAR2CSA are largely restricted to women who were infected during pregnancy. However, we discovered that VAR2CSA antibodies can also be elicited by P. vivax Duffy binding protein (PvDBP). We proposed that infection with P. vivax in non-pregnant individuals can generate antibodies that cross-react with VAR2CSA. To better understand the specificity of these antibodies, we took advantage of a mouse monoclonal antibody (3D10) raised against PvDBP that cross-reacts with VAR2CSA and identified the epitopes targeted by this antibody. We screened two peptide arrays that span the ectodomain of VAR2CSA from the FCR3 and NF54 alleles. Based on the top epitope recognized by 3D10, we designed a 34-amino acid synthetic peptide, which we call CRP1, that maps to a highly conserved region in DBL3X. Specific lysine residues are critical for 3D10 recognition, and these same amino acids are within a previously defined chondroitin sulfate A (CSA) binding site in DBL3X. We showed by isothermal titration calorimetry that the CRP1 peptide can bind directly to CSA, and antibodies to CRP1 raised in rats significantly blocked the binding of IEs to CSA in vitro. In our Colombian cohorts of pregnant and non-pregnant individuals, at least 45% were seroreactive to CRP1. Antibody reactivities to CRP1 and the 3D10 natural epitope in PvDBP region II, subdomain 1 (SD1), were strongly correlated in both cohorts. These findings suggest that antibodies arising from PvDBP may cross-react with VAR2CSA through the epitope in CRP1 and that CRP1 could be a potential vaccine candidate to target a distinct CSA binding site in VAR2CSA.
Keywords: PvDBP; VAR2CSA; cross-reactivity; malaria; peptide; pregnancy; vaccine design.
Copyright © 2023 Iyamu, Vinals, Tornyigah, Arango, Bhat, Adra, Grewal, Martin, Maestre, Overduin, Hazes and Yanow.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ayres Pereira M., Mandel Clausen T., Pehrson C., Mao Y., Resende M., Daugaard M., et al. . (2016). Placental sequestration of plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate a on syndecan-1. PloS Pathogens 12 (8), e1005831. doi: 10.1371/journal.ppat.1005831 - DOI - PMC - PubMed
-
- Baruch D. I., Gormely J. A., Ma C., Howard R. J., Pasloske B. L. (1996). Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. U S A 93 (8), 3497–3502. doi: 10.1073/pnas.93.8.3497 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

