Effectiveness, safety, and costs of thromboprophylaxis with enoxaparin or unfractionated heparin in inpatients with obesity
- PMID: 37396589
- PMCID: PMC10313352
- DOI: 10.3389/fcvm.2023.1163684
Effectiveness, safety, and costs of thromboprophylaxis with enoxaparin or unfractionated heparin in inpatients with obesity
Abstract
Background: Obesity is a frequent and significant risk factor for venous thromboembolism (VTE) among hospitalized adults. Pharmacologic thromboprophylaxis can help prevent VTE, but real-world effectiveness, safety, and costs among inpatients with obesity are unknown.
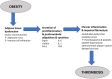
Objective: This study aims to compare clinical and economic outcomes among adult medical inpatients with obesity who received thromboprophylaxis with enoxaparin or unfractionated heparin (UFH).
Methods: A retrospective cohort study was performed using the PINC AI™ Healthcare Database, which covers more than 850 hospitals in the United States. Patients included were ≥18 years old, had a primary or secondary discharge diagnosis of obesity [International Classification of Diseases (ICD)-9 diagnosis codes 278.01, 278.02, and 278.03; ICD-10 diagnosis codes E66.0x, E66.1, E66.2, E66.8, and E66.9], received ≥1 thromboprophylactic dose of enoxaparin (≤40 mg/day) or UFH (≤15,000 IU/day) during the index hospitalization, stayed ≥6 days in the hospital, and were discharged between 01 January 2010, and 30 September 2016. We excluded surgical patients, patients with pre-existing VTE, and those who received higher (treatment-level) doses or multiple types of anticoagulants. Multivariable regression models were constructed to compare enoxaparin with UFH based on the incidence of VTE, pulmonary embolism (PE)---------related mortality, overall in-hospital mortality, major bleeding, treatment costs, and total hospitalization costs during the index hospitalization and the 90 days after index discharge (readmission period).
Results: Among 67,193 inpatients who met the selection criteria, 44,367 (66%) and 22,826 (34%) received enoxaparin and UFH, respectively, during their index hospitalization. Demographic, visit-related, clinical, and hospital characteristics differed significantly between groups. Enoxaparin during index hospitalization was associated with 29%, 73%, 30%, and 39% decreases in the adjusted odds of VTE, PE-related mortality, in-hospital mortality, and major bleeding, respectively, compared with UFH (all p < 0.002). Compared with UFH, enoxaparin was associated with significantly lower total hospitalization costs during the index hospitalization and readmission periods.
Conclusions: Among adult inpatients with obesity, primary thromboprophylaxis with enoxaparin compared with UFH was associated with significantly lower risks of in-hospital VTE, major bleeding, PE-related mortality, overall in-hospital mortality, and hospitalization costs.
Keywords: bleeding; cost analyses; enoxaparin; medical inpatients; obesity; thromboprophylaxis; unfractionated heparin; venous thromboembolism (VTE).
© 2023 Amin, Kartashov, Ngai, Steele and Rosenthal.
Conflict of interest statement
AK and NR are employees and shareholders of Premier Inc. KS and WN are employees and shareholders of Sanofi. AA has been a principal investigator or co-investigator of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, Octapharma, Fulcrum Therapeutics, Alexion and has been a speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen, La Jolla, Ferring, Seres, Spero, Eli Lilly, Gilead, Millenium, HeartRite, Aseptiscope, and Sprightly; these relationships were unrelated to the current work.
Figures
References
-
- Agency for Healthcare Research and Quality. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement. Rockville, MD (2016).
-
- Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. (1999) 341:793–800. 10.1056/NEJM199909093411103 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

