Impact of minimal residual disease standardised assessment by FDG-PET/CT in transplant-eligible patients with newly diagnosed multiple myeloma enrolled in the imaging sub-study of the FORTE trial
- PMID: 37396807
- PMCID: PMC10314158
- DOI: 10.1016/j.eclinm.2023.102017
Impact of minimal residual disease standardised assessment by FDG-PET/CT in transplant-eligible patients with newly diagnosed multiple myeloma enrolled in the imaging sub-study of the FORTE trial
Abstract
Background: 18F-FDG-PET/CT is the current standard technique to define minimal residual disease (MRD) outside the bone marrow (BM) in multiple myeloma (MM), recently standardised applying the Deauville scores (DS) to focal lesions (FS) and bone marrow uptake (BMS) and defining the complete metabolic response (CMR) as uptake below the liver background (DS <4).
Methods: In this analysis, we aimed at confirming the role of CMR, and complementarity with BM multiparameter flow cytometry (MFC) at 10-5, in an independent cohort of newly diagnosed transplant-eligible MM patients previously enrolled in the phase II randomised FORTE trial. 109 of the 474 global patients enrolled in the trial between February 23, 2015, and April 5, 2017, who had paired PET/CT (performed at baseline [B] and preceding maintenance therapy [PM]) and MFC evaluation, were included in this analysis.
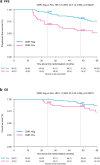
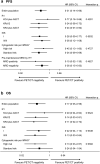
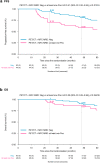
Findings: At B, 93% of patients had focal lesions within the bones (FS ≥4 in 89%) and 99% increased BM uptake (BMS ≥4 in 61%). At PM, CMR was achieved in 63% of patients, which was a strong predictor for prolonged PFS in univariate analysis at landmark time PM (HR 0.40, P = 0.0065) and in Cox multivariate analysis (HR 0.31, P = 0.0023). Regarding OS, a trend in favour of CMR was present in univariate (HR 0.44, P = 0.094), and Cox multivariate model (HR 0.17, P = 0.0037). Patients achieving both PET/CT CMR and MFC negativity at PM showed significantly extended PFS in univariate (HR 0.45, P = 0.020) and multivariate analysis (HR 0.41, P = 0.015).
Interpretation: We herein confirm the applicability and validity of DS criteria to define CMR and its prognostic relevance and complementarity with MFC at the BM level.
Funding: Amgen, Celgene/Bristol Myers Squibb, Italian Ministry of Health (RC-2022-2773423).
Keywords: Complete metabolic response (CMR); FDG-PET/CT; Minimal residual disease (MRD); Multiparameter flow cytometry (MFC); Newly diagnosed multiple myeloma (NDMM).
© 2023 The Author(s).
Conflict of interest statement
EZ receives honoraria from Janssen, Bristol-Myers Squibb, Amgen, Takeda. SO has received honoraria from Amgen, Celgene/Bristol Myers Squibb, and Janssen; has served on the advisory boards for Adaptive Biotechnologies, Janssen, Amgen, and Takeda. FG has received honoraria from Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, AbbVie, and GlaxoSmithKline; has served on the advisory boards for Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, AbbVie, GlaxoSmithKline, Roche, Adaptive Biotechnologies, Oncopeptides, bluebird bio, and Pfizer. MD has received honoraria for lectures from GlaxoSmithKline, Sanofi, and Janssen; has served on the advisory boards for GlaxoSmithKline, Sanofi, and Bristol Myers Squibb. AB has served on the advisory board for Amgen, Janssen, Takeda, Celgene, GlaxoSmithKline. MGa has received honoraria from Janssen, Bristol-Myers Squibb, Amgen, Takeda, GlaxoSmithKline. RZ has served on the advisory boards for Amgen, Celgene, Janssen, Takeda, Bristol Myers Squibb, GlaxoSmithKline, and Pfizer. BG has received honoraria from Amgen, Bristol Myers Squibb, Janssen, and Takeda; has served on the advisory boards for Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, and Takeda. AV received honoraria from Novartis, Advanced Accelerator Applications and GE Healthcare. FP receives honoraria and travel accommodation support from Celgene, Janssen, Takeda and has served on the advisory boards for Celgene BMS, Janssen, Amgen, GlaxoSmithKline. PT has received honoraria from Janssen, Celgene, Bristol Myers Squibb, Amgen, Takeda, AbbVie, Sanofi, GlaxoSmithKline, and Pfizer; has served on the data safety monitoring boards or advisory boards for Janssen, Celgene, Bristol Myers Squibb, and Amgen. KM has received honoraria from Celgene, Takeda, Amgen, Sanofi, Janssen. MB has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and AbbVie; has served on the advisory boards for Janssen and GlaxoSmithKline; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and Mundipharma. PM has received honoraria from and/or served on scientific boards for AbbVie, Alexion, Amgen, AstraZeneca, Astellas, BeiGene, Bristol-Myers Squibb/Celgene, Gilead, GlaxoSmithKline, Incyte, Janssen, Jazz, Novartis, Pfizer, Roche, Sanofi, and Takeda. MC has received honoraria from Janssen, Celgene, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Takeda, AbbVie, Sanofi, Pfizer, and Adaptive Biotechnologies; has served on the advisory boards for Janssen, Bristol Myers Squibb, Sanofi, Amgen, GlaxoSmithKline, and Pfizer; has served on the speakers’ bureaus for Janssen, Celgene, and Sanofi. CN has been PET revisor for Keosys-Sanofi. The other authors declare no competing financial interests.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

