Endoglin and squamous cell carcinomas
- PMID: 37396898
- PMCID: PMC10313935
- DOI: 10.3389/fmed.2023.1112573
Endoglin and squamous cell carcinomas
Abstract
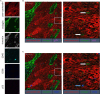
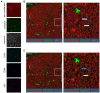
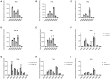
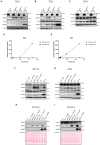
Despite the fact that the role of endoglin on endothelial cells has been extensively described, its expression and biological role on (epithelial) cancer cells is still debatable. Especially its function on squamous cell carcinoma (SCC) cells is largely unknown. Therefore, we investigated SCC endoglin expression and function in three types of SCCs; head and neck (HNSCC), esophageal (ESCC) and vulvar (VSCC) cancers. Endoglin expression was evaluated in tumor specimens and 14 patient-derived cell lines. Next to being expressed on angiogenic endothelial cells, endoglin is selectively expressed by individual SCC cells in tumor nests. Patient derived HNSCC, ESCC and VSCC cell lines express varying levels of endoglin with high interpatient variation. To assess the function of endoglin in signaling of TGF-β ligands, endoglin was overexpressed or knocked out or the signaling was blocked using TRC105, an endoglin neutralizing antibody. The endoglin ligand BMP-9 induced strong phosphorylation of SMAD1 independent of expression of the type-I receptor ALK1. Interestingly, we observed that endoglin overexpression leads to strongly increased soluble endoglin levels, which in turn decreases BMP-9 signaling. On the functional level, endoglin, both in a ligand dependent and independent manner, did not influence proliferation or migration of the SCC cells. In conclusion, these data show endoglin expression on individual cells in the tumor nests in SCCs and a role for (soluble) endoglin in paracrine signaling, without directly affecting proliferation or migration in an autocrine manner.
Keywords: BMP-9; TGF-β; TRC105; endoglin; squamous cell carcinoma.
Copyright © 2023 Hakuno, Janson, Trietsch, de Graaf, de Jonge-Muller, Crobach, Harryvan, Boonstra, Dinjens, Slingerland and Hawinkels.
Conflict of interest statement
SH was employed by InnoSer België NV, outside the submitted work. LH reports sponsored research grants from TRACON Pharmaceuticals, not related to the work in this study. In addition, LH is coinventor on a patent on the combination of TRC105 with PD1 therapy issued to TRACON. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Kurn H, Daly DT. Histology. Treasure Island (FL): Epithelial Cell. Statpearls; (2022).
LinkOut - more resources
Full Text Sources
Research Materials

