Apolipoprotein A1 is associated with osteocalcin and bone mineral density rather than high-density lipoprotein cholesterol in Chinese postmenopausal women with type 2 diabetes mellitus
- PMID: 37396919
- PMCID: PMC10308019
- DOI: 10.3389/fmed.2023.1182866
Apolipoprotein A1 is associated with osteocalcin and bone mineral density rather than high-density lipoprotein cholesterol in Chinese postmenopausal women with type 2 diabetes mellitus
Abstract
Objective: Disturbances in high-density lipoprotein cholesterol (HDL-c) metabolic pathways can affect bone metabolism, which may rely on the particle function of apolipoprotein rather than HDL-c levels. This study aimed to evaluate the correlation of serum HDL-c and apolipoprotein A1 (APOA1) with bone metabolism in Chinese postmenopausal women with type 2 diabetes mellitus (T2DM).
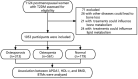
Method: A total of 1,053 participants with complete data were enrolled and separated into three groups based on the HDL-c and APOA1 tertiles. The trained reviewer collected demographic and anthropometric information. Bone turnover markers (BTMs) were determined by standard methods. Bone mineral density (BMD) was measured by dual-energy x-ray absorptiometry.
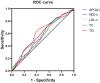
Results: Overall, the prevalence of osteoporosis was 29.7%. Groups with higher APOA1 have a remarkably more elevated level of osteocalcin (OC), L1-L4 BMD, and T-score across the APOA1 tertiles. APOA1 presented a positive correlation with OC (r = 0.194, p < 0.001), L1-L4 BMD (r = 0.165, p < 0.001), and T-score (r = 0.153, p < 0.001) rather than HDL-c. Meanwhile, APOA1 remained independently associated with OC (β = 0.126, p < 0.001), L1-L4 BMD (β = 0.181, p < 0.001), and T-score (β = 0.180, p < 0.001) after adjustment for confounding factors. APOA1 is also shown to be independently correlated with osteoporosis after adjustment for confounding factors, and the OR (95%CI) was 0.851 (0.784-0.924). In contrast, there was no significant association between HDL-c and osteoporosis. Furthermore, APOA1 seemed to have the largest areas under the curve (AUC) for osteoporosis. The AUC (95% CI) of APOA1 identifying osteoporosis was 0.615 (0.577-0.652). The optimal cut-off value of APOA1 was 0.89 g/L (sensitivity: 56.5%, specificity: 67.9%).
Conclusion: APOA1 is independently associated with OC, L1-L4 BMD, and osteoporosis rather than HDL-c in Chinese postmenopausal women with T2DM.
Keywords: apolipoprotein A1; bone mineral density; bone turnover markers; high-density lipoprotein; osteoporosis.
Copyright © 2023 Wang, Chen, Lv, Tu and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The diagnostic value of the combined application of blood lipid metabolism markers and interleukin-6 in osteoporosis and osteopenia.Lipids Health Dis. 2025 Feb 5;24(1):38. doi: 10.1186/s12944-025-02456-2. Lipids Health Dis. 2025. PMID: 39910539 Free PMC article.
-
Association of apolipoprotein A1 levels with lumbar bone mineral density and β-CTX in osteoporotic fracture individuals: a cross-sectional investigation.Front Med (Lausanne). 2024 Jul 31;11:1415739. doi: 10.3389/fmed.2024.1415739. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39144661 Free PMC article.
-
Monocyte to high-density lipoprotein and apolipoprotein A1 ratios are associated with bone homeostasis imbalance caused by chronic inflammation in postmenopausal women with type 2 diabetes mellitus.Front Pharmacol. 2022 Nov 7;13:1062999. doi: 10.3389/fphar.2022.1062999. eCollection 2022. Front Pharmacol. 2022. PMID: 36419622 Free PMC article.
-
Perirenalfat thickness is associated with bone turnover markers and bone mineral density in postmenopausal women with type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2022 Oct 25;13:990667. doi: 10.3389/fendo.2022.990667. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387843 Free PMC article.
-
HDL cholesterol and bone mineral density: is there a genetic link?Bone. 2012 Feb;50(2):525-33. doi: 10.1016/j.bone.2011.07.002. Bone. 2012. PMID: 21810493 Free PMC article. Review.
Cited by
-
The diagnostic value of the combined application of blood lipid metabolism markers and interleukin-6 in osteoporosis and osteopenia.Lipids Health Dis. 2025 Feb 5;24(1):38. doi: 10.1186/s12944-025-02456-2. Lipids Health Dis. 2025. PMID: 39910539 Free PMC article.
-
U-shaped association between TC/HDL-C ratio and osteoporosis risk in older adults.Sci Rep. 2025 Feb 8;15(1):4791. doi: 10.1038/s41598-025-89537-5. Sci Rep. 2025. PMID: 39922960 Free PMC article.
-
Association of apolipoprotein A1 levels with lumbar bone mineral density and β-CTX in osteoporotic fracture individuals: a cross-sectional investigation.Front Med (Lausanne). 2024 Jul 31;11:1415739. doi: 10.3389/fmed.2024.1415739. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39144661 Free PMC article.
-
Proteomic Analysis of Human Serum Proteins Adsorbed onto Collagen Barrier Membranes.J Funct Biomater. 2024 Oct 9;15(10):302. doi: 10.3390/jfb15100302. J Funct Biomater. 2024. PMID: 39452600 Free PMC article.
-
Nonlinear relationship between lumbar bone mineral density and high-density lipoprotein cholesterol in patients with type 2 diabetes.Front Endocrinol (Lausanne). 2025 Jun 2;16:1570841. doi: 10.3389/fendo.2025.1570841. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40529826 Free PMC article.
References
-
- Sato M, Ye W, Sugihara T, Isaka Y. Fracture risk and healthcare resource utilization and costs among osteoporosis patients with type 2 diabetes mellitus and without diabetes mellitus in Japan: retrospective analysis of a hospital claims database. BMC Musculoskelet Disord. (2016) 17:489. doi: 10.1186/s12891-016-1344-9, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

