Remote Ischemic Conditioning in Pediatric Cancer Patients Receiving Anthracycline Chemotherapy: A Sham-Controlled Single-Blind Randomized Trial
- PMID: 37397078
- PMCID: PMC10308057
- DOI: 10.1016/j.jaccao.2022.11.020
Remote Ischemic Conditioning in Pediatric Cancer Patients Receiving Anthracycline Chemotherapy: A Sham-Controlled Single-Blind Randomized Trial
Abstract
Background: Anthracycline cardiotoxicity is a concern in survivors of childhood cancers. Recent evidence suggests that remote ischemic conditioning (RIC) may offer myocardial protection.
Objectives: This randomized sham-controlled single-blind study tested the hypothesis that RIC may reduce myocardial injury in pediatric cancer patients receiving anthracycline chemotherapy.
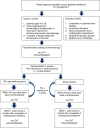
Methods: We performed a phase 2 sham-controlled single-blind randomized controlled trial to determine the impact of RIC on myocardial injury in pediatric cancer patients receiving anthracycline-based chemotherapy. Patients were randomized to receive RIC (3 cycles of 5-minute inflation of a blood pressure cuff placed over 1 limb to 15 mm Hg above systolic pressure) or sham intervention. The intervention was applied within 60 minutes before initiation of the first dose and before up to 4 cycles of anthracycline therapy. The primary outcome was the plasma high-sensitivity cardiac troponin T (hs-cTnT) level. The secondary outcome measures included echocardiographic indexes of left ventricular systolic and diastolic function and the occurrence of cardiovascular events.
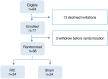
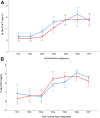
Results: A total of 68 children 10.9 ± 3.9 years of age were randomized to receive RIC (n = 34) or sham (n = 34) intervention. Plasma levels of hs-cTnT showed a progressive increase across time points in the RIC (P < 0.001) and sham (P < 0.001) groups. At each of the time points, there were no significant differences in hs-cTnT levels or LV tissue Doppler and strain parameters between the 2 groups (all P > 0.05). None of the patients developed heart failure or cardiac arrhythmias.
Conclusions: RIC did not exhibit cardioprotective effects in childhood cancer patients receiving anthracycline-based chemotherapy. (Remote Ischaemic Preconditioning in Childhood Cancer [RIPC]; NCT03166813).
Keywords: anthracycline; pediatric cancer; remote ischemic conditioning.
© 2023 The Authors.
Conflict of interest statement
This study was supported by the Health and Health Services Research Fund, Food and Health Bureau, Hong Kong SAR Government (grant 05161506). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



Comment in
-
Remote Ischemic Conditioning for Anthracycline Cardiotoxicity: The Need to Protect the Most Vulnerable.JACC CardioOncol. 2023 Jun 20;5(3):356-359. doi: 10.1016/j.jaccao.2023.05.002. eCollection 2023 Jun. JACC CardioOncol. 2023. PMID: 37397082 Free PMC article.
References
-
- Gatta G., Capocaccia R., Coleman M.P., Ries L.A., Berrino F. Childhood cancer survival in Europe and the United States. Cancer. 2002;95:1767–1772. - PubMed
-
- Kremer L.C., van Dalen E.C., Offringa M., Ottenkamp J., Voûte P.A. Anthracyline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19:191–196. - PubMed
-
- Lipshultz S.E., Lipsitz S.R., Sallan S.E., et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. - PubMed
-
- Lipshultz S.E., Sallan S.E., Giantris A.L., Lipsitz S.R., Dalton V., Colan S.D. 48 h continuous doxorubicin infusion is not cardioprotective in children assessed 18 months later: the DFCI 91001 ALL protocol. Proc Am Soc Clin Oncol. 1998;17:528a.
-
- Lipshultz S.E., Giantris A.L., Lipsitz S.R., et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20:1677–1682. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

