A highly sensitive electrochemical sensor by growing Ag nanoparticles on the surface of PPy@PEDOT:PSS film for detecting sodium hydroxymethanesulfinate molecules
- PMID: 37397227
- PMCID: PMC10314181
- DOI: 10.1016/j.fochx.2023.100701
A highly sensitive electrochemical sensor by growing Ag nanoparticles on the surface of PPy@PEDOT:PSS film for detecting sodium hydroxymethanesulfinate molecules
Abstract
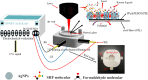
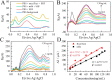
A high-sensitivity electrochemical sensor was fabricated via in situ growth of Ag nanoparticles (AgNPs) on the surface of a polypyrrole@poly(3,4-ethylenedioxythiophene):polystyrene sulfonic acid (PPy@PEDOT:PSS) film for detecting sodium hydroxymethanesulfinate (SHF) molecules in milk and rice flour samples. The sensor fabrication process involved randomly decorating Ag seed points on the porous PPy@PEDOT:PSS film via a chemical reduction process using a AgNO3 solution. Next, AgNPs were anchored on the PPy@PEDOT:PSS film surface using an electrochemical deposition method to prepare a sensor electrode. Under optimal conditions, the sensor exhibits a good linear relation within a range of 1-130 ng/mL for real milk and rice flour samples and its limit-of-detection values were up to 0.58 and 0.29 ng/mL, respectively. Additionally, Raman spectroscopy was used to identify the byproducts of the chemical reaction, such as formaldehyde. This AgNP/PPy@PEDOT:PSS film-based electrochemical sensor offers a simple and rapid method for detecting SHF molecules in food products.
Keywords: Ag nanoparticles (AgNPs); Differential pulse voltammetry; Electrochemical sensor; Milk matrix; Polypyrrole@Poly(3,4-ethylenedioxythiophene)-poly (styrenesulfonate); Raman spectroscopy; Sodium hydroxymethanesulfinate.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
A Multilayer Interlaced Ag Nanosheet Film Prepared by an Electrodeposition Method on a PPy@PEDOT:PSS Film: A Strategy to Prepare Sensitive Surface-Enhanced Raman Scattering Substrates.ACS Omega. 2022 Mar 8;7(11):9380-9387. doi: 10.1021/acsomega.1c06387. eCollection 2022 Mar 22. ACS Omega. 2022. PMID: 35350326 Free PMC article.
-
Electrochemical Sensors based on Gold-Silver Core-Shell Nanoparticles Combined with a Graphene/PEDOT:PSS Composite Modified Glassy Carbon Electrode for Paraoxon-ethyl Detection.ACS Omega. 2024 Jan 2;9(2):2896-2910. doi: 10.1021/acsomega.3c08349. eCollection 2024 Jan 16. ACS Omega. 2024. PMID: 38250352 Free PMC article.
-
Unveiling the PEDOT-polypyrrole hybrid electrode for the electrochemical sensing of dopamine.Sci Rep. 2025 Mar 31;15(1):10989. doi: 10.1038/s41598-024-82355-1. Sci Rep. 2025. PMID: 40164631 Free PMC article.
-
Thermoelectric Properties of Flexible PEDOT:PSS/Polypyrrole/Paper Nanocomposite Films.Materials (Basel). 2017 Jul 11;10(7):780. doi: 10.3390/ma10070780. Materials (Basel). 2017. PMID: 28773141 Free PMC article.
-
Polypyrrole-poly(3,4-ethylenedioxythiophene)-Ag (PPy-PEDOT-Ag) nanocomposite films for label-free electrochemical DNA sensing.Biosens Bioelectron. 2013 Sep 15;47:133-40. doi: 10.1016/j.bios.2013.02.049. Epub 2013 Mar 21. Biosens Bioelectron. 2013. PMID: 23578969
Cited by
-
Recent advances in ratiometric electrochemical sensors for food analysis.Food Chem X. 2024 Jul 23;23:101681. doi: 10.1016/j.fochx.2024.101681. eCollection 2024 Oct 30. Food Chem X. 2024. PMID: 39157660 Free PMC article. Review.
References
-
- Abedalwafa M.A., Tang Z., Qiao Y., Mei Q., Yang G., Li Y., Wang L.J.M.A. An aptasensor strip-based colorimetric determination method for kanamycin using cellulose acetate nanofibers decorated DNA–gold nanoparticle bioconjugates. Microchimica Acta. 2020;187:1–9. doi: 10.1007/s00604-020-04348-x. - DOI - PubMed
-
- Bavandpour R., Karimi-Maleh H., Asif M., Gupta V.K., Atar N., Abbasghorbani M.J.J. Liquid phase determination of adrenaline uses a voltammetric sensor employing CuFe2O4 nanoparticles and room temperature ionic liquids. Journal of Molecular Liquids. 2016;213:369–373. doi: 10.1016/j.molliq.2015.07.054. - DOI
-
- Chapman E., Barinaga C., Udseth H., Smith R. Confirmation and quantitation of hydroxymethanesulfonate in precipitation by electrospray ionization-tandem mass spectrometry. Atmospheric Environment Part A. General Topics. 1990;24(12):2951–2957. doi: 10.1016/0960-1686(90)90475-3. - DOI
-
- Dong Q., Ryu H., Lei Y.J.E.A. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochimica Acta. 2021;370 doi: 10.1016/j.electacta.2021.137744. - DOI
-
- Dovrou E., Lim C.Y., Canagaratna M.R., Kroll J.H., Worsnop D.R., Keutsch F.N. Measurement techniques for identifying and quantifying hydroxymethanesulfonate (HMS) in an aqueous matrix and particulate matter using aerosol mass spectrometry and ion chromatography. Atmospheric Measurement Techniques. 2019;12(10):5303–5315. doi: 10.5194/amt-12-5303-2019. - DOI
LinkOut - more resources
Full Text Sources

