Current status of artificial intelligence analysis for the treatment of pancreaticobiliary diseases using endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography
- PMID: 37397344
- PMCID: PMC10312781
- DOI: 10.1002/deo2.267
Current status of artificial intelligence analysis for the treatment of pancreaticobiliary diseases using endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography
Abstract
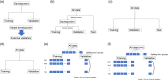
Pancreatic and biliary diseases encompass a range of conditions requiring accurate diagnosis for appropriate treatment strategies. This diagnosis relies heavily on imaging techniques like endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography. Artificial intelligence (AI), including machine learning and deep learning, is becoming integral in medical imaging and diagnostics, such as the detection of colorectal polyps. AI shows great potential in diagnosing pancreatobiliary diseases. Unlike machine learning, which requires feature extraction and selection, deep learning can utilize images directly as input. Accurate evaluation of AI performance is a complex task due to varied terminologies, evaluation methods, and development stages. Essential aspects of AI evaluation involve defining the AI's purpose, choosing appropriate gold standards, deciding on the validation phase, and selecting reliable validation methods. AI, particularly deep learning, is increasingly employed in endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography diagnostics, achieving high accuracy levels in detecting and classifying various pancreatobiliary diseases. The AI often performs better than doctors, even in tasks like differentiating benign from malignant pancreatic tumors, cysts, and subepithelial lesions, identifying gallbladder lesions, assessing endoscopic retrograde cholangiopancreatography difficulty, and evaluating the biliary strictures. The potential for AI in diagnosing pancreatobiliary diseases, especially where other modalities have limitations, is considerable. However, a crucial constraint is the need for extensive, high-quality annotated data for AI training. Future advances in AI, such as large language models, promise further applications in the medical field.
Keywords: ERCP; EUS; artificial intelligence; deep learning; pancreas.
© 2023 The Authors. DEN Open published by John Wiley & Sons Australia, Ltd on behalf of Japan Gastroenterological Endoscopy Society.
Conflict of interest statement
Takamichi Kuwahara, Kazuo Hara, Shin Haba, Nozomi Okuno, Toshitaka Fukui, Minako Urata, and Yoshitaro Yamamoto declare no conflict of interest related to this study. Nobumasa Mizuno has received Grants or contracts from any entity from to their institution from Novartis, MSD, Incyte, Ono Pharmaceutical, Seagen, Dainippon Sumitomo Pharma; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Yakult Honsha, AstraZeneca, Novartis, FUJIFILM Toyama Chemical, MSD, Taiho Pharmaceutical; and has participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca.
Figures


References
-
- WHO Classification of Tumours Editorial Board . Digestive System Tumors. WHO Classification of Tumors, 5th edn, Lyon: IARC Press, 2019.
-
- Lloyd RV, Osamura R, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs, 4th edn, Lyon: IARC Press, 2017.
-
- Okazaki K, Chari ST, Frulloni L et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology 2017; 17: 1–6. - PubMed
-
- Tanaka M, Fernández‐del Castillo C, Kamisawa T et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017; 17: 738–53. - PubMed
-
- Kuwahara T, Hara K, Mizuno N et al. Current status of artificial intelligence analysis for endoscopic ultrasonography. Dig Endosc 2021; 33: 298–305. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
