Real-world data of pyrotinib-based therapy for patients with brain metastases of HER2-positive advanced breast cancer: a single-center retrospective analysis and molecular portraits
- PMID: 37397372
- PMCID: PMC10313114
- DOI: 10.3389/fonc.2023.1105474
Real-world data of pyrotinib-based therapy for patients with brain metastases of HER2-positive advanced breast cancer: a single-center retrospective analysis and molecular portraits
Abstract
Introduction: Pyrotinib is a novel irreversible pan-HER tyrosine kinase inhibitor (TKI). However, real-world data of pyrotinib-containing therapy in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) and developing brain metastases (BMs) are limited, and the genomic profile of this subpopulation is almost undefined.
Methods and materials: Patients with BM of HER2-positive MBC (n = 35) treated with pyrotinib-containing therapy were enrolled in this analysis. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and toxicity profiles were evaluated. Hazard ratios (HRs) and 95% confidence intervals (CIs) for disease progression were estimated using the Cox proportional hazards models. Targeted next-generation sequencing of 618 cancer-relevant genes was performed on plasma and primary breast tumors from patients with BM and without BM.
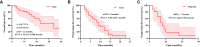
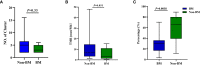
Results: The median PFS time was 8.00 (95% CI, 5.98-10.017) months, and the median OS time was 23 (95% CI, 10.412-35.588) months. The ORR was 45.7%, and the DCR was 74.3%. In the Cox multivariate analysis, prior exposure to brain radiotherapy (HR = 3.268), received pyrotinib as third- or higher-line treatment (HR = 4.949), subtentorial brain metastasis (HR = 6.222), and both supratentorial and subtentorial brain metastases (HR = 5.863) were independently associated with increased risk of progression. The frequent grade 3-4 adverse event was increased direct bilirubin (14.3%), and two patients suffered from grade 3-4 diarrhea. In the exploratory genomic analysis, altered frequencies of FGFR3, CD276, CDC73, and EPHX1 were higher in the BM group. The consistency of mutated profiles of plasma and primary lesion in the BM group was significantly lower (30.4% vs. 65.5%; p = 0.0038).
Conclusions: Pyrotinib-containing therapy shows favorable effectiveness and tolerable safety in patients with BM of HER2-positive MBC, particularly in a population that is brain radiotherapy-naïve, received pyrotinib as first- or second-line treatment, and developed supratentorial brain metastasis. In the exploratory genomic analysis, patients with BM showed distinct genomic features from patients without BM.
Keywords: HER2-positive breast cancer; brain metastases; clinical benefits; genomic profile; pyrotinib.
Copyright © 2023 Wang, Liu, Zhang, Zhang, Ran, Ma, Yang, Wang, He, Zhao, Wang, Zhang, Dong and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Crivellari D, Gelber RD, Simoncini E, Zahrieh D, Goldhirsch A, Murray E, et al. . Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the international breast cancer study group (IBCSG). Ann Oncol (2006) 17(6):935–44. doi: 10.1093/annonc/mdl064 - DOI - PubMed
-
- Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, et al. . Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol (2006) 24(36):5658–63. doi: 10.1200/JCO.2006.07.0250 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

