Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study
- PMID: 37397389
- PMCID: PMC10312386
- DOI: 10.3389/fonc.2023.1075823
Metformin is associated with improved clinical outcomes in patients with melanoma: a retrospective, multi-institutional study
Abstract
Background: Pre-clinical studies have shown that metformin reduces intratumoral hypoxia, improves T-cell function, and increases sensitivity to PD-1 blockade, and metformin exposure has been associated with improved clinical outcomes in various types of cancer. However, the impact of this drug in diabetic melanoma patients has not yet been fully elucidated.
Methods: We reviewed 4,790 diabetic patients with stage I-IV cutaneous melanoma treated at the UPMC-Hillman Cancer Center and Memorial Sloan Kettering Cancer Center between 1996-2020. The primary endpoints included recurrence rates, progression free survival (PFS), and overall survival (OS) with and without metformin exposure. Tabulated variables included BRAF mutational status, immunotherapy (IMT) by type, and incidence of brain metastases.
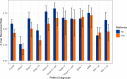
Results: The five-year incidence of recurrence in stage I/II patients was significantly reduced with metformin exposure (32.3% vs 47.7%, p=0.012). The five-year recurrence rate for stage III patients was also significantly reduced (58.3% vs 77.3%, p=0.013) in the metformin cohort. OS was numerically increased in nearly all stages exposed to metformin, though this did not reach statistical significance. The incidence of brain metastases was significantly lower in the metformin cohort (8.9% vs 14.6%, p=0.039).
Conclusion: This is the first study to demonstrate significantly improved clinical outcomes in diabetic melanoma patients exposed to metformin. Overall, these results provide further rationale for ongoing clinical trials studying the potential augmentation of checkpoint blockade with metformin in advanced melanoma.
Keywords: checkpoint blockade; melanoma; metformin; oxidative phosphorylation; progression-free survival; tumor infiltrating lymphocyte; tumor microenvironment (TME).
Copyright © 2023 Augustin, Huang, Ding, Zhai, McArdle, Santisi, Davis, Sander, Davar, Kirkwood, Delgoffe, Warner and Najjar.
Conflict of interest statement
DD: Grant/research support: Bristol Meyers Squibb, Checkmate, Merck, GlaskoSmith Kline, Tesaro, Amgen Honoraria: GlaxoSmith Kline, Tesaro, Instil Bio, Array Biopharma. Advisory board: GlaxoSmith Kline. JK: Grant/research support and/or consultant: BMS, Merck, Novartis, Roche, Genentech, EMD, Serono, Array Biopharma, Prometheus. GD: Grant/research support: Pfizer, Bluebirdbio, TCR2 Therapeutics. Consultant: TTMS, Pieris Pharmaceuticals. AW: Consulting/advisory: Nanobiotix, Iovance, Novartis, Shanghai Jo’Ann Medical Technology, BluePath Solutions, Pfizer, Instil Bio. YGN: Grant/research support: Merck, Bristol Meyers Squibb, Pfizer, Replimune. Consulting/Advisory board: Array Biopharma, InterVenn Bio, Merck, Novartis, Pfizer, BMS and Immunocore. Non-CE Honoraria: Pfizer, Immuncore. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Melanoma of the skin {{/amp]]mdash; cancer stat facts. Available at: https://seer.cancer.gov/statfacts/html/melan.html (Accessed October 22, 2021).
-
- Robert C, Ribas A, Schachter J, Arance A, Grob J-J, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol (2019) 20(9):1239–51. doi: 10.1016/S1470-2045(19)30388-2 - DOI - PubMed
-
- Eggermont AMM, Blank CU, Mandalà M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol (2021) 22(5):643–54. doi: 10.1016/S1470-2045(21)00065-6 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials