Ginseng saponin metabolite 20(S)-protopanaxadiol relieves pulmonary fibrosis by multiple-targets signaling pathways
- PMID: 37397411
- PMCID: PMC10310916
- DOI: 10.1016/j.jgr.2023.01.002
Ginseng saponin metabolite 20(S)-protopanaxadiol relieves pulmonary fibrosis by multiple-targets signaling pathways
Abstract
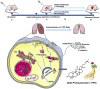
Background: Panax ginseng Meyer is a representative Chinese herbal medicine with antioxidant and anti-inflammatory activity. 20(S)-Protopanaxadiol (PPD) has been isolated from ginseng and shown to have promising pharmacological activities. However, effects of PDD on pulmonary fibrosis (PF) have not been reported. We hypothesize that PDD may reverse inflammation-induced PF and be a novel therapeutic strategy.
Methods: Adult male C57BL/6 mice were used to establish a model of PF induced by bleomycin (BLM). The pulmonary index was measured, and histological and immunohistochemical examinations were made. Cell cultures of mouse alveolar epithelial cells were analyzed with Western blotting, co-immunoprecipitation, immunofluorescence, immunohistochemistry, siRNA transfection, cellular thermal shift assay and qRT-PCR.
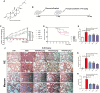
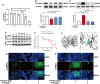
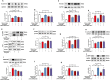
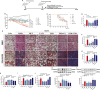
Results: The survival rate of PPD-treated mice was higher than that of untreated BLM-challenged mice. Expression of fibrotic hallmarks, including α-SMA, TGF-β1 and collagen I, was reduced by PPD treatment, indicating attenuation of PF. Mice exposed to BLM had higher STING levels in lung tissue, and this was reduced by phosphorylated AMPK after activation by PPD. The role of phosphorylated AMPK in suppressing STING was confirmed in TGF-β1-incubated cells. Both in vivo and in vitro analyses indicated that PPD treatment attenuated BLM-induced PF by modulating the AMPK/STING signaling pathway.
Conclusion: PPD ameliorated BLM-induced PF by multi-target regulation. The current study may help develop new therapeutic strategies for preventing PF.
Keywords: 20(S)-protopanaxadiol; Adenosine 5'monophosphate-activated protein kinase; Pulmonary fibrosis; Stimulator of interferon genes.
© 2023 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures


Similar articles
-
The dual role of 20(S)-protopanaxadiol in alleviating pulmonary fibrosis through the gut-lung axis.Phytomedicine. 2024 Jul;129:155699. doi: 10.1016/j.phymed.2024.155699. Epub 2024 May 1. Phytomedicine. 2024. PMID: 38733907
-
Juglanin alleviates bleomycin-induced lung injury by suppressing inflammation and fibrosis via targeting sting signaling.Biomed Pharmacother. 2020 Jul;127:110119. doi: 10.1016/j.biopha.2020.110119. Epub 2020 Apr 7. Biomed Pharmacother. 2020. PMID: 32276127
-
Protective effects of heterophyllin B against bleomycin-induced pulmonary fibrosis in mice via AMPK activation.Eur J Pharmacol. 2022 Apr 15;921:174825. doi: 10.1016/j.ejphar.2022.174825. Epub 2022 Mar 10. Eur J Pharmacol. 2022. PMID: 35283110
-
BTB and CNC homology 1 inhibition ameliorates fibrosis and inflammation via blocking ERK pathway in pulmonary fibrosis.Exp Lung Res. 2021 Feb-Mar;47(2):67-77. doi: 10.1080/01902148.2020.1849448. Epub 2020 Nov 26. Exp Lung Res. 2021. PMID: 33238752
-
Follistatin-Like 1 Promotes Bleomycin-Induced Pulmonary Fibrosis through the Transforming Growth Factor Beta 1/Mitogen-Activated Protein Kinase Signaling Pathway.Chin Med J (Engl). 2018 Aug 20;131(16):1917-1925. doi: 10.4103/0366-6999.238151. Chin Med J (Engl). 2018. PMID: 30082522 Free PMC article.
Cited by
-
Advances in cGAS-STING Signaling in Fibrosis Diseases: Therapeutic Target in Pathological Scars.J Inflamm Res. 2025 Aug 9;18:10777-10793. doi: 10.2147/JIR.S541656. eCollection 2025. J Inflamm Res. 2025. PMID: 40809466 Free PMC article. Review.
-
The role of cGAS-STING signaling in pulmonary fibrosis and its therapeutic potential.Front Immunol. 2023 Oct 25;14:1273248. doi: 10.3389/fimmu.2023.1273248. eCollection 2023. Front Immunol. 2023. PMID: 37965345 Free PMC article. Review.
-
cGAS-STING targeting offers therapy choice in lung diseases.Biol Direct. 2025 Feb 7;20(1):20. doi: 10.1186/s13062-025-00611-4. Biol Direct. 2025. PMID: 39920718 Free PMC article. Review.
-
Progress of cGAS-STING signaling pathway-based modulation of immune response by traditional Chinese medicine in clinical diseases.Front Immunol. 2024 Dec 16;15:1510628. doi: 10.3389/fimmu.2024.1510628. eCollection 2024. Front Immunol. 2024. PMID: 39737190 Free PMC article. Review.
References
-
- Tao N., Li K., Liu J., Fan G., Sun T. Liproxstatin-1 alleviates bleomycin-induced alveolar epithelial cells injury and mice pulmonary fibrosis via attenuating inflammation, reshaping redox equilibrium, and suppressing ROS/p53/α-SMA pathway. Biochem Biophys Res Commun. 2021;551:133–139. doi: 10.1016/j.bbrc.2021.02.127. - DOI - PubMed
-
- Liu J., Peng D., You J., Zhou O., Qiu H., Hao C., et al. Type 2 alveolar epithelial cells differentiated from human umbilical cord mesenchymal Stem cells alleviate mouse pulmonary fibrosis through β-catenin-regulated cell apoptosis. Stem Cells Dev. 2021;30(13):660–670. doi: 10.1089/scd.2020.0208. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

