Emergent structural and functional properties of hippocampal multi-cellular aggregates
- PMID: 37397454
- PMCID: PMC10311220
- DOI: 10.3389/fnins.2023.1171115
Emergent structural and functional properties of hippocampal multi-cellular aggregates
Abstract
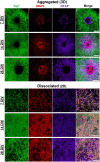
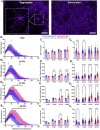
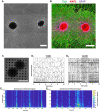
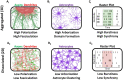
Hippocampal neural networks are distinctly capable of integrating multi-modal sensory inputs to drive memory formation. Neuroscientific investigations using simplified in vitro models have greatly relied on planar (2D) neuronal cultures made from dissociated tissue. While these models have served as simple, cost-effective, and high-throughput tools for examining various morphological and electrophysiological characteristics of hippocampal networks, 2D cultures fail to reconstitute critical elements of the brain microenvironment that may be necessary for the emergence of sophisticated integrative network properties. To address this, we utilized a forced aggregation technique to generate high-density (>100,000 cells/mm3) multi-cellular three-dimensional aggregates using rodent embryonic hippocampal tissue. We contrasted the emergent structural and functional properties of aggregated (3D) and dissociated (2D) cultures over 28 days in vitro (DIV). Hippocampal aggregates displayed robust axonal fasciculation across large distances and significant neuronal polarization, i.e., spatial segregation of dendrites and axons, at earlier time points compared to dissociated cultures. Moreover, we found that astrocytes in aggregate cultures self-organized into non-overlapping quasi-domains and developed highly stellate morphologies resembling astrocyte structures in vivo. We maintained cultures on multi-electrode arrays (MEAs) to assess spontaneous electrophysiological activity for up to 28 DIV. We found that 3D networks of aggregated cultures developed highly synchronized networks and with high burstiness by 28 DIV. We also demonstrated that dual-aggregate networks became active by 7 DIV, in contrast to single-aggregate networks which became active and developed synchronous bursting activity with repeating motifs by 14 DIV. Taken together, our findings demonstrate that the high-density, multi-cellular, 3D microenvironment of hippocampal aggregates supports the recapitulation of emergent biofidelic morphological and functional properties. Our findings suggest that neural aggregates may be used as segregated, modular building blocks for the development of complex, multi-nodal neural network topologies.
Keywords: 3D neuron cultures; In vitro hippocampal culture; astrocyte domains; in vitro electrophysiology; neural spheroid; neuronal polarization.
Copyright © 2023 Acero, Das, Rivellini, Purvis, Adewole and Cullen.
Conflict of interest statement
DC was a scientific co-founder of Innervace, Inc., and Axonova Medical, LLC, which are University of Pennsylvania spin-out companies focused in neuroregenerative medicine. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
3-D multi-electrode arrays detect early spontaneous electrophysiological activity in 3-D neuronal-astrocytic co-cultures.Biomed Eng Lett. 2020 Jul 31;10(4):579-591. doi: 10.1007/s13534-020-00166-5. eCollection 2020 Nov. Biomed Eng Lett. 2020. PMID: 33194249 Free PMC article.
-
Interfacing 3D Engineered Neuronal Cultures to Micro-Electrode Arrays: An Innovative In Vitro Experimental Model.J Vis Exp. 2015 Oct 18;(105):e53080. doi: 10.3791/53080. J Vis Exp. 2015. PMID: 26554533 Free PMC article.
-
Rapid generation of functional engineered 3D human neuronal assemblies: network dynamics evaluated by micro-electrodes arrays.J Neural Eng. 2021 Dec 10;18(6). doi: 10.1088/1741-2552/ac3e02. J Neural Eng. 2021. PMID: 34844234
-
Spontaneous Cell Cluster Formation in Human iPSC-Derived Neuronal Spheroid Networks Influences Network Activity.eNeuro. 2022 Oct 10;9(5):ENEURO.0143-22.2022. doi: 10.1523/ENEURO.0143-22.2022. Online ahead of print. eNeuro. 2022. PMID: 36216508 Free PMC article.
-
A new indirect co-culture set up of mouse hippocampal neurons and cortical astrocytes on microelectrode arrays.J Neurosci Methods. 2012 Mar 15;204(2):262-72. doi: 10.1016/j.jneumeth.2011.11.030. Epub 2011 Dec 13. J Neurosci Methods. 2012. PMID: 22182586
References
Grants and funding
LinkOut - more resources
Full Text Sources

