Licraside as novel potent FXR agonist for relieving cholestasis: structure-based drug discovery and biological evaluation studies
- PMID: 37397498
- PMCID: PMC10309033
- DOI: 10.3389/fphar.2023.1197856
Licraside as novel potent FXR agonist for relieving cholestasis: structure-based drug discovery and biological evaluation studies
Abstract
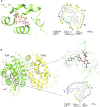
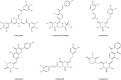
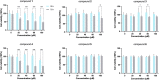
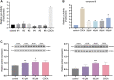
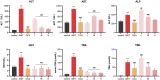
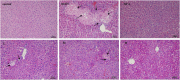
Cholestasis is a common clinical disease caused by a disorder in bile acids (BAs) homeostasis, which promotes its development. The Farnesoid X receptor (FXR) plays a critical role in regulating BAs homeostasis, making it an essential target for cholestasis treatment. Although several active FXR agonists have been identified, effective drugs for cholestasis are still lacking. To address this, a molecular docking-based virtual screening method was used to identify potential FXR agonists. A hierarchical screening strategy was employed to improve the screening accuracy, and six compounds were selected for further evaluation. Dual-luciferase reporter gene assay was used to demonstrate FXR activation by the screened compounds, and their cytotoxicity was then evaluated. Among the compounds, licraside showed the best performance and was selected for in vivo evaluation using an ANIT-induced cholestasis animal model. Results demonstrated that licraside significantly reduced biliary TBA, serum ALT, AST, GGT, ALP, TBIL, and TBA levels. Liver histopathological analysis showed that licraside also had a therapeutic effect on ANIT-induced liver injury. Overall, these findings suggest that licraside is an FXR agonist with potential therapeutic effects on cholestasis. This study provides valuable insights into the development of novel lead compounds from traditional Chinese medicine for cholestasis treatment.
Keywords: FXR; agonist; biological evaluation; cholestasis; licraside; virtual screening.
Copyright © 2023 Xi, Shi, Shen, Wang, Wei and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Alisma orientale extract exerts the reversing cholestasis effect by activation of farnesoid X receptor.Phytomedicine. 2018 Mar 15;42:34-42. doi: 10.1016/j.phymed.2018.03.017. Epub 2018 Mar 8. Phytomedicine. 2018. PMID: 29655695
-
Yangonin protects against cholestasis and hepatotoxity via activation of farnesoid X receptor in vivo and in vitro.Toxicol Appl Pharmacol. 2018 Jun 1;348:105-116. doi: 10.1016/j.taap.2018.04.015. Epub 2018 Apr 14. Toxicol Appl Pharmacol. 2018. PMID: 29660435
-
The discovery of a new potent FXR agonist based on natural product screening.Bioorg Chem. 2024 Feb;143:106979. doi: 10.1016/j.bioorg.2023.106979. Epub 2023 Nov 19. Bioorg Chem. 2024. PMID: 37995646
-
Targeting FXR in Cholestasis.Handb Exp Pharmacol. 2019;256:299-324. doi: 10.1007/164_2019_231. Handb Exp Pharmacol. 2019. PMID: 31201556 Review.
-
Nuclear receptor FXR, bile acids and liver damage: Introducing the progressive familial intrahepatic cholestasis with FXR mutations.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1308-1318. doi: 10.1016/j.bbadis.2017.09.019. Epub 2017 Sep 29. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28965883 Review.
Cited by
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Apr 26;9(1):97. doi: 10.1038/s41392-024-01811-6. Signal Transduct Target Ther. 2024. PMID: 38664391 Free PMC article. Review.
-
Insights into the gut-liver axis: mechanisms and emerging therapies in hepatocellular carcinoma.Front Pharmacol. 2025 May 19;16:1595853. doi: 10.3389/fphar.2025.1595853. eCollection 2025. Front Pharmacol. 2025. PMID: 40458800 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

