This is a preprint.
Comparison of genomic diversity between single and pooled Staphylococcus aureus colonies isolated from human colonisation cultures
- PMID: 37397999
- PMCID: PMC10312683
- DOI: 10.1101/2023.06.14.544959
Comparison of genomic diversity between single and pooled Staphylococcus aureus colonies isolated from human colonisation cultures
Update in
-
Comparison of genomic diversity between single and pooled Staphylococcus aureus colonies isolated from human colonization cultures.Microb Genom. 2023 Nov;9(11):001111. doi: 10.1099/mgen.0.001111. Microb Genom. 2023. PMID: 37934072 Free PMC article.
Abstract
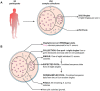
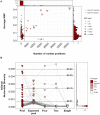
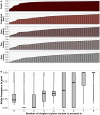
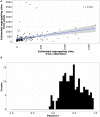
The most common approach to sampling the bacterial populations within an infected or colonised host is to sequence genomes from a single colony obtained from a culture plate. However, it is recognized that this method does not capture the genetic diversity in the population. An alternative is to sequence a mixture containing multiple colonies ("pool-seq"), but this has the disadvantage that it is a non-homogeneous sample, making it difficult to perform specific experiments. We compared differences in measures of genetic diversity between eight single-colony isolates (singles) and pool-seq on a set of 2286 S. aureus culture samples. The samples were obtained by swabbing three body sites on 85 human participants quarterly for a year, who initially presented with a methicillin-resistant S. aureus skin and soft-tissue infection (SSTI). We compared parameters such as sequence quality, contamination, allele frequency, nucleotide diversity and pangenome diversity in each pool to the corresponding singles. Comparing singles from the same culture plate, we found that 18% of sample collections contained mixtures of multiple Multilocus sequence types (MLSTs or STs). We showed that pool-seq data alone could predict the presence of multi-ST populations with 95% accuracy. We also showed that pool-seq could be used to estimate the number of polymorphic sites in the population. Additionally, we found that the pool may contain clinically relevant genes such as antimicrobial resistance markers that may be missed when only examining singles. These results highlight the potential advantage of analysing genome sequences of total populations obtained from clinical cultures rather than single colonies.
Keywords: Staphylococcus aureus; adaptation; asymptomatic carriage; genetic diversity; whole genome sequencing.
Conflict of interest statement
Conflict of interest statement The authors declare no conflict of interest.
Figures



References
-
- Talbot BM, Jacko NF, Petit RA III, Pegues DA, Shumaker MJ, Read TD, et al. Unsuspected Clonal Spread of Methicillin-Resistant Staphylococcus aureus Causing Bloodstream Infections in Hospitalized Adults Detected Using Whole Genome Sequencing. Clin Infect Dis. 2022. Dec 15;75(12):2104–12. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
