This is a preprint.
Single-cell type analysis of wing premotor circuits in the ventral nerve cord of Drosophila melanogaster
- PMID: 37398009
- PMCID: PMC10312520
- DOI: 10.1101/2023.05.31.542897
Single-cell type analysis of wing premotor circuits in the ventral nerve cord of Drosophila melanogaster
Abstract
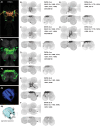
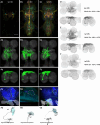
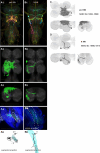
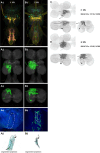
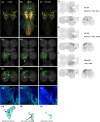
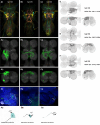
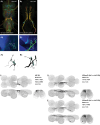
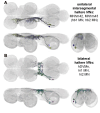
To perform most behaviors, animals must send commands from higher-order processing centers in the brain to premotor circuits that reside in ganglia distinct from the brain, such as the mammalian spinal cord or insect ventral nerve cord. How these circuits are functionally organized to generate the great diversity of animal behavior remains unclear. An important first step in unraveling the organization of premotor circuits is to identify their constituent cell types and create tools to monitor and manipulate these with high specificity to assess their functions. This is possible in the tractable ventral nerve cord of the fly. To generate such a toolkit, we used a combinatorial genetic technique (split-GAL4) to create 195 sparse transgenic driver lines targeting 196 individual cell types in the ventral nerve cord. These included wing and haltere motoneurons, modulatory neurons, and interneurons. Using a combination of behavioral, developmental, and anatomical analyses, we systematically characterized the cell types targeted in our collection. In addition, we identified correspondences between the cells in this collection and a recent connectomic data set of the ventral nerve cord. Taken together, the resources and results presented here form a powerful toolkit for future investigations of neuronal circuits and connectivity of premotor circuits while linking them to behavioral outputs.
Keywords: Drosophila; Split-GAL4; courtship song; flight; motoneuron; ventral nerve cord; wing.
Figures

























































Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Comparative connectomics of Drosophila descending and ascending neurons.Nature. 2025 Jul;643(8070):158-172. doi: 10.1038/s41586-025-08925-z. Epub 2025 Apr 30. Nature. 2025. PMID: 40307549 Free PMC article.
-
A library of lineage-specific driver lines connects developing neuronal circuits to behavior in the Drosophila Ventral Nerve Cord.bioRxiv [Preprint]. 2025 Apr 28:2024.11.27.625713. doi: 10.1101/2024.11.27.625713. bioRxiv. 2025. Update in: Elife. 2025 Jun 10;14:RP106042. doi: 10.7554/eLife.106042. PMID: 39651218 Free PMC article. Updated. Preprint.
-
Interventions for central serous chorioretinopathy: a network meta-analysis.Cochrane Database Syst Rev. 2025 Jun 16;6(6):CD011841. doi: 10.1002/14651858.CD011841.pub3. Cochrane Database Syst Rev. 2025. PMID: 40522203
Cited by
-
Wings of Change: aPKC/FoxP-dependent plasticity in steering motor neurons underlies operant self-learning in Drosophila.F1000Res. 2024 Jun 11;13:116. doi: 10.12688/f1000research.146347.2. eCollection 2024. F1000Res. 2024. PMID: 38779314 Free PMC article.
References
-
- AGEE H. R. & ORONA E. 1988. Studies of the Neural Basis of Evasive Flight Behavior in Response to Acoustic Stimulation in Heliothis zea (Lepidoptera: Noctuidae): Organization of the Tympanic Nerves. Annals of the Entomological Society of America, 81, 977–985.
-
- ANDO N., WANG H., SHIRAI K., KIGUCHI K. & KANZAKI R. 2011. Central projections of the wing afferents in the hawkmoth, Agrius convolvuli. J Insect Physiol, 57, 1518–36. - PubMed
-
- ARBER S. 2012. Motor Circuits in Action: Specification, Connectivity, and Function. Neuron, 74, 975–989. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
