This is a preprint.
Combination of Polymeric Micelle Formulation of TGFβ Receptor Inhibitors and Paclitaxel Produce Consistent Response Across Different Mouse Models of TNBC
- PMID: 37398150
- PMCID: PMC10312717
- DOI: 10.1101/2023.06.14.544381
Combination of Polymeric Micelle Formulation of TGFβ Receptor Inhibitors and Paclitaxel Produce Consistent Response Across Different Mouse Models of TNBC
Update in
-
Combination of polymeric micelle formulation of TGFβ receptor inhibitors and paclitaxel produces consistent response across different mouse models of Triple-negative breast cancer.Bioeng Transl Med. 2024 Jun 4;9(5):e10681. doi: 10.1002/btm2.10681. eCollection 2024 Sep. Bioeng Transl Med. 2024. PMID: 39553439 Free PMC article.
Abstract
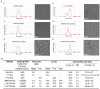
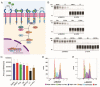
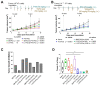
Triple-negative breast cancer (TNBC) is notoriously difficult to treat due to the lack of targetable receptors and sometimes poor response to chemotherapy. The transforming growth factor-beta (TGFβ) family of proteins and their receptors (TGFR) are highly expressed in TNBC and implicated in chemotherapy-induced cancer stemness. Here we evaluated combination treatments using experimental TGFR inhibitors (TGFβi), SB525334 (SB), and LY2109761 (LY) with Paclitaxel (PTX) chemotherapy. These TGFβi target TGFR-I (SB) or both TGFR-I&II (LY). Due to the poor water solubility of these drugs, we incorporated each of them in poly(2-oxazoline) (POx) high-capacity polymeric micelles (SB-POx and LY-POx). We assessed their anti-cancer effect as single agents and in combination with micellar Paclitaxel (PTX-POx) using multiple immunocompetent TNBC mouse models that mimic human subtypes (4T1, T11-Apobec and T11-UV). While either TGFβi or PTX showed a differential effect in each model as single agents, the combinations were consistently effective against all three models. Genetic profiling of the tumors revealed differences in the expression levels of genes associated with TGFβ, EMT, TLR-4, and Bcl2 signaling, alluding to the susceptibility to specific gene signatures to the treatment. Taken together, our study suggests that TGFβi and PTX combination therapy using high-capacity POx micelle delivery provides a robust anti-tumor response in multiple TNBC subtype mouse models.
Conflict of interest statement
Conflict of Interest: A.V.K. is an inventor on patents pertinent to the subject matter of the present contribution, co-founder, stockholder and director of DelAqua Pharmaceuticals Inc. having intent of commercial development of POx-based drug formulations. A.V.K. is also a co-founder, stockholder and director of SoftKemo Pharma Corp. and BendaRx Pharma Corp., which develop polymeric drug formulation and a blood cancer drug. M.S.P. discloses potential interest in DelAqua Pharmaceuticals Inc., SoftKemo Pharma Corp. and BendaRx Pharma Corp. as a spouse of a co-founder. C.M.P is an equity stockholder and consultant of BioClassifier LLC; C.M.P is also listed as an inventor on patent applications for the Breast PAM50 Subtyping assay.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA: a cancer journal for clinicians 71(3) (2021) 209–249. - PubMed
-
- Fornier M., Fumoleau P., The paradox of triple negative breast cancer: novel approaches to treatment, The breast journal 18(1) (2012) 41–51. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
