This is a preprint.
SCS: cell segmentation for high-resolution spatial transcriptomics
- PMID: 37398213
- PMCID: PMC10312435
- DOI: 10.1101/2023.01.11.523658
SCS: cell segmentation for high-resolution spatial transcriptomics
Update in
-
SCS: cell segmentation for high-resolution spatial transcriptomics.Nat Methods. 2023 Aug;20(8):1237-1243. doi: 10.1038/s41592-023-01939-3. Epub 2023 Jul 10. Nat Methods. 2023. PMID: 37429992
Abstract
Spatial transcriptomics promises to greatly improve our understanding of tissue organization and cell-cell interactions. While most current platforms for spatial transcriptomics only offer multi-cellular resolution, with 10-15 cells per spot, recent technologies provide a much denser spot placement leading to sub-cellular resolution. A key challenge for these newer methods is cell segmentation and the assignment of spots to cells. Traditional image-based segmentation methods are limited and do not make full use of the information profiled by spatial transcrip-tomics. Here we present SCS, which combines imaging data with sequencing data to improve cell segmentation accuracy. SCS assigns spots to cells by adaptively learning the position of each spot relative to the center of its cell using a transformer neural network. SCS was tested on two new sub-cellular spatial transcriptomics technologies and outperformed traditional image-based segmentation methods. SCS achieved better accuracy, identified more cells, and provided more realistic cell size estimation. Sub-cellular analysis of RNAs using SCS spots assignments provides information on RNA localization and further supports the segmentation results.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures
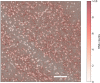
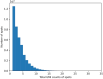
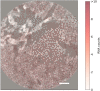
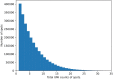




References
-
- Ståhl Patrik L, Salmén Fredrik, Vickovic Sanja, Lundmark Anna, Navarro José Fernández, Magnusson Jens, Giacomello Stefania, Asp Michaela, Westholm Jakub O, Huss Mikael, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science, 353(6294):78–82, 2016. - PubMed
-
- Chen Ao, Liao Sha, Cheng Mengnan, Ma Kailong, Wu Liang, Lai Yiwei, Qiu Xiaojie, Yang Jin, Xu Jiangshan, Hao Shijie, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using dna nanoball-patterned arrays. Cell, 185(10):1777–1792, 2022. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
