This is a preprint.
Rescue of secretion of a rare-disease associated mis-folded mutant glycoprotein in UGGT1 knock-out mammalian cells
- PMID: 37398215
- PMCID: PMC10312515
- DOI: 10.1101/2023.05.30.542711
Rescue of secretion of a rare-disease associated mis-folded mutant glycoprotein in UGGT1 knock-out mammalian cells
Update in
-
Rescue of secretion of rare-disease-associated misfolded mutant glycoproteins in UGGT1 knock-out mammalian cells.Traffic. 2024 Jan;25(1):e12927. doi: 10.1111/tra.12927. Traffic. 2024. PMID: 38272446 Free PMC article.
Abstract
Endoplasmic reticulum (ER) retention of mis-folded glycoproteins is mediated by the ERlocalised eukaryotic glycoprotein secretion checkpoint, UDP-glucose glycoprotein glucosyl-transferase (UGGT). The enzyme recognises a mis-folded glycoprotein and flags it for ER retention by reglucosylating one of its N-linked glycans. In the background of a congenital mutation in a secreted glycoprotein gene, UGGT-mediated ER retention can cause rare disease even if the mutant glycoprotein retains activity ("responsive mutant"). Here, we investigated the subcellular localisation of the human Trop-2 Q118E variant, which causes gelatinous droplike corneal dystrophy (GDLD). Compared with the wild type Trop-2, which is correctly localised at the plasma membrane, the Trop-2-Q118E variant is found to be heavily retained in the ER. Using Trop-2-Q118E, we tested UGGT modulation as a rescue-of-secretion therapeutic strategy for congenital rare disease caused by responsive mutations in genes encoding secreted glycoproteins. We investigated secretion of a EYFP-fusion of Trop-2-Q118E by confocal laser scanning microscopy. As a limiting case of UGGT inhibition, mammalian cells harbouring CRISPR/Cas9-mediated inhibition of the UGGT1 and/or UGGT2 gene expressions were used. The membrane localisation of the Trop-2-Q118E-EYFP mutant was successfully rescued in UGGT1-/- and UGGT1/2-/- cells. UGGT1 also efficiently reglucosylated Trop-2-Q118E-EYFP in cellula. The study supports the hypothesis that UGGT1 modulation constitutes a novel therapeutic strategy for the treatment of Trop-2-Q118E associated GDLD, and it encourages the testing of modulators of ER glycoprotein folding Quality Control (ERQC) as broad-spectrum rescueof-secretion drugs in rare diseases caused by responsive secreted glycoprotein mutants.
Keywords: GDLD; TACSTD2; Trop-2; UGGT; UGGT1; UGGT2; glycoprotein secretion; responsive mutant.
Figures
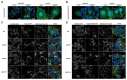
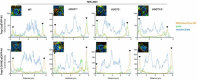
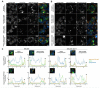


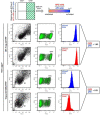
References
-
- Klausner RD, Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990;62: 611–614. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
