This is a preprint.
Changes in the Type 2 diabetes gut mycobiome associate with metformin treatment across populations
- PMID: 37398234
- PMCID: PMC10312434
- DOI: 10.1101/2023.05.25.542255
Changes in the Type 2 diabetes gut mycobiome associate with metformin treatment across populations
Update in
-
Changes in the type 2 diabetes gut mycobiome associate with metformin treatment across populations.mBio. 2024 Jun 12;15(6):e0016924. doi: 10.1128/mbio.00169-24. Epub 2024 May 20. mBio. 2024. PMID: 38767350 Free PMC article.
Abstract
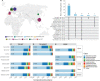
The human gut teems with a diverse ecosystem of microbes, yet non-bacterial portions of that community are overlooked in studies of metabolic diseases firmly linked to gut bacteria. Type 2 diabetes mellitus (T2D) associates with compositional shifts in the gut bacterial microbiome and fungal mycobiome, but whether T2D and/or pharmaceutical treatments underpin the community change is unresolved. To differentiate these effects, we curated a gut mycobiome cohort to-date spanning 1,000 human samples across 5 countries and a murine experimental model. We use Bayesian multinomial logistic normal models to show that metformin and T2D both associate with shifts in the relative abundance of distinct gut fungi. T2D associates with shifts in the Saccharomycetes and Sordariomycetes fungal classes, while the genera Fusarium and Tetrapisipora most consistently associate with metformin treatment. We confirmed the impact of metformin on individual gut fungi by administering metformin to healthy mice. Thus, metformin and T2D account for subtle, but significant and distinct variation in the gut mycobiome across human populations. This work highlights for the first time that oral pharmaceuticals can confound associations of gut fungi with T2D and warrants the need to consider pharmaceutical interventions in investigations of linkages between metabolic diseases and gut microbial inhabitants.
Keywords: Gut mycobiome; gut microbiome; metagenomics; type 2 diabetes mellitus.
Conflict of interest statement
Disclosure Statement: The authors report there are no competing interests to declare.
Figures



References
-
- Zhang F., Aschenbrenner D., Yoo J. Y. & Zuo T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 3, e969–e983 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
