This is a preprint.
Phage predation, disease severity and pathogen genetic diversity in cholera patients
- PMID: 37398242
- PMCID: PMC10312676
- DOI: 10.1101/2023.06.14.544933
Phage predation, disease severity and pathogen genetic diversity in cholera patients
Update in
-
Phage predation, disease severity, and pathogen genetic diversity in cholera patients.Science. 2024 Apr 19;384(6693):eadj3166. doi: 10.1126/science.adj3166. Epub 2024 Apr 19. Science. 2024. PMID: 38669570
Abstract
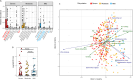
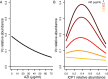
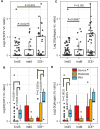
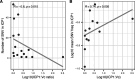
Despite an increasingly detailed picture of the molecular mechanisms of phage-bacterial interactions, we lack an understanding of how these interactions evolve and impact disease within patients. Here we report a year-long, nation-wide study of diarrheal disease patients in Bangladesh. Among cholera patients, we quantified Vibrio cholerae (prey) and its virulent phages (predators) using metagenomics and quantitative PCR, while accounting for antibiotic exposure using quantitative mass spectrometry. Virulent phage (ICP1) and antibiotics suppressed V. cholerae to varying degrees and were inversely associated with severe dehydration depending on resistance mechanisms. In the absence of anti-phage defenses, predation was 'effective,' with a high predator:prey ratio that correlated with increased genetic diversity among the prey. In the presence of anti-phage defenses, predation was 'ineffective,' with a lower predator:prey ratio that correlated with increased genetic diversity among the predators. Phage-bacteria coevolution within patients should therefore be considered in the deployment of phage-based therapies and diagnostics.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures

References
-
- Cholera – Global situation (2023). WHO Report. (available at https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON437).
-
- Islam M. S. et al., Microbiological investigation of diarrhoea epidemics among Rwandan refugees in Zaire. Trans R Soc Trop Med Hyg 89, 506 (1995). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
