This is a preprint.
MFGE8 inhibits insulin signaling through PTP1B
- PMID: 37398282
- PMCID: PMC10312531
- DOI: 10.1101/2023.05.30.542928
MFGE8 inhibits insulin signaling through PTP1B
Update in
-
PTP1B mediates the inhibitory effect of MFGE8 on insulin signaling through the β5 integrin.J Biol Chem. 2024 Feb;300(2):105631. doi: 10.1016/j.jbc.2024.105631. Epub 2024 Jan 8. J Biol Chem. 2024. PMID: 38199575 Free PMC article.
Abstract
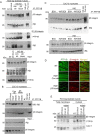
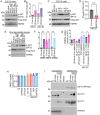
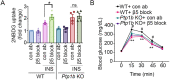
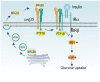
The role of integrins in regulating insulin signaling is incompletely understood. We have previously shown that binding of the integrin ligand milk fat globule epidermal growth factor like 8 (MFGE8) to the αvβ5 integrin promotes termination of insulin receptor signaling in mice. Upon ligation of MFGE8, β5 complexes with the insulin receptor beta (IRβ) in skeletal muscle resulting in dephosphorylation of IRβ and reduction of insulin-stimulated glucose uptake. Here we investigate the mechanism by which the interaction between β5 and IRβ impacts IRβ phosphorylation status. We show that β5 blockade inhibits and MFGE8 promotes PTP1B binding to and dephosphorylation of IRβ resulting in reduced or increased insulin-stimulated myotube glucose uptake respectively. The β5-PTP1B complex is recruited by MFGE8 to IRβ leading to termination of canonical insulin signaling. β5 blockade enhances insulin-stimulated glucose uptake in wild type but not Ptp1b KO mice indicating that PTP1B functions downstream of MFGE8 in modulating insulin receptor signaling. Furthermore, in a human cohort, we report serum MFGE8 levels correlate with indices of insulin resistance. These data provide mechanistic insights into the role of MFGE8 and β5 in regulating insulin signaling.
Conflict of interest statement
Conflict of interest None
Figures
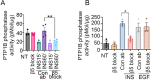

References
-
- Datta R., Gholampour M. A., Yang C. D., Volk R., Lin S., Podolsky M. J., Arnold T., Rieder F., Zaro B. W., Verzi M., Lehner R., Abumrad N., Lizama C. O., and Atabai K. (2023) MFGE8 links absorption of dietary fatty acids with catabolism of enterocyte lipid stores through HNF4gamma-dependent transcription of CES enzymes. Cell Rep 42, 112249. - PMC - PubMed
-
- Datta R., Lizama C. O., Soltani A. K., McKleroy W., Podolsky M. J., Yang C. D., Huynh T. L., Cautivo K. M., Wang B., Koliwad S. K., Abumrad N. A., and Atabai K. (2021) Autoregulation of insulin receptor signaling through MFGE8 and the alphavbeta5 integrin. Proceedings of the National Academy of Sciences of the United States of America 118 - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
