This is a preprint.
Evaluating the Performance of the Astral Mass Analyzer for Quantitative Proteomics Using Data Independent Acquisition
- PMID: 37398334
- PMCID: PMC10312564
- DOI: 10.1101/2023.06.03.543570
Evaluating the Performance of the Astral Mass Analyzer for Quantitative Proteomics Using Data Independent Acquisition
Update in
-
Evaluating the Performance of the Astral Mass Analyzer for Quantitative Proteomics Using Data-Independent Acquisition.J Proteome Res. 2023 Oct 6;22(10):3290-3300. doi: 10.1021/acs.jproteome.3c00357. Epub 2023 Sep 8. J Proteome Res. 2023. PMID: 37683181 Free PMC article.
Abstract
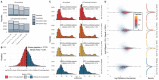
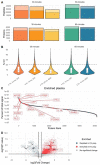
We evaluate the quantitative performance of the newly released Asymmetric Track Lossless (Astral) analyzer. Using data independent acquisition, the Thermo Scientific™ Orbitrap™ Astral™ mass spectrometer quantifies 5 times more peptides per unit time than state-of-the-art Thermo Scientific™ Orbitrap™ mass spectrometers, which have long been the gold standard for high resolution quantitative proteomics. Our results demonstrate that the Orbitrap Astral mass spectrometer can produce high quality quantitative measurements across a wide dynamic range. We also use a newly developed extra-cellular vesicle enrichment protocol to reach new depths of coverage in the plasma proteome, quantifying over 5,000 plasma proteins in a 60-minute gradient with the Orbitrap Astral mass spectrometer.
Conflict of interest statement
Notes
The authors declare the following competing financial interest(s): The MacCoss Lab at the University of Washington has a sponsored research agreement with Thermo Fisher Scientific, the manufacturer of the instrumentation used in this research. However, analytical techniques were selected and performed independent of Thermo Fisher Scientific. M.J.M. is a paid consultant for Thermo Fisher Scientific. E.D.,T.N.A., A.C.P., E.D., J.P., A.P., P.M.R., M.W.S., H.I.S., C.H., A.A.M., D.H., and V.Z. are employees of Thermo Fisher Scientific, the manufacturer of the instrumentation used in this research.
Figures


References
-
- Brunner A.-D.; Thielert M.; Vasilopoulou C. G.; Ammar C.; Coscia F.; Mund A.; Hoerning O. B.; Bache N.; Apalategui A.; Lubeck M.; Richter S.; Fischer D. S.; Raether O.; Park M. A.; Meier F.; Theis F. J.; Mann M. Ultra-High Sensitivity Mass Spectrometry Quantifies Single-Cell Proteome Changes upon Perturbation; preprint; Systems Biology, 2020. 10.1101/2020.12.22.423933. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
