This is a preprint.
Modelling Human Post-Implantation Development via Extra-Embryonic Niche Engineering
- PMID: 37398391
- PMCID: PMC10312773
- DOI: 10.1101/2023.06.15.545118
Modelling Human Post-Implantation Development via Extra-Embryonic Niche Engineering
Update in
-
Modelling post-implantation human development to yolk sac blood emergence.Nature. 2024 Feb;626(7998):367-376. doi: 10.1038/s41586-023-06914-8. Epub 2023 Dec 13. Nature. 2024. PMID: 38092041 Free PMC article.
Abstract
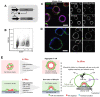
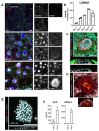
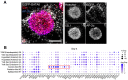
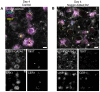
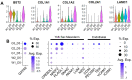
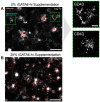
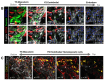
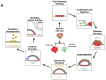
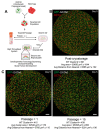
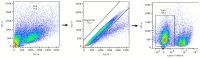
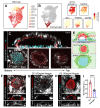
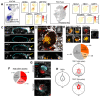
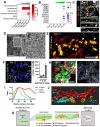
Implantation of the human embryo commences a critical developmental stage that comprises profound morphogenetic alteration of embryonic and extra-embryonic tissues, axis formation, and gastrulation events. Our mechanistic knowledge of this window of human life remains limited due to restricted access to in vivo samples for both technical and ethical reasons. Additionally, human stem cell models of early post-implantation development with both embryonic and extra-embryonic tissue morphogenesis are lacking. Here, we present iDiscoid, produced from human induced pluripotent stem cells via an engineered a synthetic gene circuit. iDiscoids exhibit reciprocal co-development of human embryonic tissue and engineered extra-embryonic niche in a model of human post-implantation. They exhibit unanticipated self-organization and tissue boundary formation that recapitulates yolk sac-like tissue specification with extra-embryonic mesoderm and hematopoietic characteristics, the formation of bilaminar disc-like embryonic morphology, the development of an amniotic-like cavity, and acquisition of an anterior-like hypoblast pole and posterior-like axis. iDiscoids offer an easy-to-use, high-throughput, reproducible, and scalable platform to probe multifaceted aspects of human early post-implantation development. Thus, they have the potential to provide a tractable human model for drug testing, developmental toxicology, and disease modeling.
Conflict of interest statement
Competing Interests: The authors declare no conflicts of interest. J.H., S.K., and M.R.E. have filed for IP for the technology presented here.
Figures












References
Methods References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
