This is a preprint.
GENE EXPRESSION WITHIN A HUMAN CHOROIDAL NEOVASCULAR MEMBRANE USING SPATIAL TRANSCRIPTOMICS
- PMID: 37398429
- PMCID: PMC10312719
- DOI: 10.1101/2023.06.16.544770
GENE EXPRESSION WITHIN A HUMAN CHOROIDAL NEOVASCULAR MEMBRANE USING SPATIAL TRANSCRIPTOMICS
Update in
-
Gene Expression Within a Human Choroidal Neovascular Membrane Using Spatial Transcriptomics.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):40. doi: 10.1167/iovs.64.13.40. Invest Ophthalmol Vis Sci. 2023. PMID: 37878301 Free PMC article.
Abstract
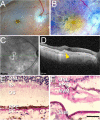
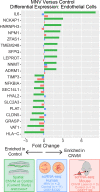
Macular neovascularization is a relatively common and potentially visually devastating complication of age-related macular degeneration. In macular neovascularization, pathologic angiogenesis can originate from either the choroid or the retina, but we have limited understanding of how different cell types become dysregulated in this dynamic process. In this study, we performed spatial RNA sequencing on a human donor eye with macular neovascularization as well as a healthy control donor. We identified genes enriched within the area of macular neovascularization and used deconvolution algorithms to predict the originating cell type of these dysregulated genes. Within the area of neovascularization, endothelial cells were predicted to increase expression of genes related to Rho family GTPase signaling and integrin signaling. Likewise, VEGF and TGFB1 were identified as potential upstream regulators that could drive the observed gene expression changes produced by endothelial and retinal pigment epithelium cells in the macular neovascularization donor. These spatial gene expression profiles were compared to previous single-cell gene expression experiments in human age-related macular degeneration as well as a model of laser-induced neovascularization in mice. As a secondary aim, we also investigated spatial gene expression patterns within the macular neural retina and between the macular and peripheral choroid. We recapitulated previously described regional-specific gene expression patterns across both tissues. Overall, this study spatially analyzes gene expression across the retina, retinal pigment epithelium, and choroid in health and describes a set of candidate molecules that become dysregulated in macular neovascularization.
Keywords: AMD; choroid; gene expression; neovascularization; spatial RNA sequencing.
Conflict of interest statement
CONFLICT OF INTEREST: No authors declare competing interests.
Figures




References
-
- Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, Souied EH, Cohen SY, Querques G, Ohno-Matsui K, Boyer D et al., Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 127, 616–636 (2020). - PMC - PubMed
-
- Golbaz I, Ahlers C, Stock G, Schütze C, Schriefl S, Schlanitz F, Simader C, Prünte C, Schmidt-Erfurth UM, Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial compartments in exudative AMD during anti-VEGF therapy. Investigative ophthalmology & visual science 52, 1599–1605 (2011). - PubMed
-
- Keane PA, Liakopoulos S, Ongchin SC, Heussen FM, Msutta S, Chang KT, Walsh AC, Sadda SR, Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Investigative ophthalmology & visual science 49, 3115–3120 (2008). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
