Residual insomnia in major depressive disorder: a systematic review
- PMID: 37398584
- PMCID: PMC10312086
- DOI: 10.3389/fpsyt.2023.1190415
Residual insomnia in major depressive disorder: a systematic review
Abstract
Introduction: The ultimate goal in major depressive disorder (MDD) treatment is recovery. A proportion of MDD patients with formal remission experience persistent difficulties, which impair their daily functioning. Residual insomnia is one of the most common residual symptoms. Patients with residual insomnia experience relapse significantly earlier and have a poor prognosis. Little is known about possible ways of treatment and what subtype of insomnia is mostly reported.
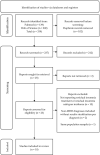
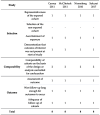
Methods: A systematic literature review was carried out in PubMed and Web of Science to synthesize the current status of knowledge about effective treatment methods and insomnia subtypes in residual insomnia in MDD.
Results: A few non-pharmacological treatment methods e.g., Cognitive Behavioral Therapy for Insomnia (CBT-I), Mindfulness-Based Cognitive Therapy (MBCT), behavioral activation (BA) and pharmacological methods (gabapentin, clonazepam) have proven to mitigate residual insomnia. Cognitive Behavioral Therapy for Depression (CBT-D) ameliorates insomnia complaints to a limited extent. Mid-nocturnal insomnia is the most common residual insomnia subtype in MDD patients.
Conclusion: Residual insomnia is a very common complaint and most often appears as mid-nocturnal insomnia. Scarce data points out the benefits from pharmacotherapy, psychotherapy, and BA. More research is needed.
Keywords: behavioral activation; cognitive-behavioral therapy; depression; major depressive disorder; psychopharmacology; psychotherapy; residual insomnia; residual symptoms.
Copyright © 2023 Kwaśny, Włodarczyk, Dywel, Szarmach, Strandberg and Cubała.
Conflict of interest statement
AK has received research support from MSD. AW and JS has received research support from Actavis, Eli Lilly, Minerva Neurosciences, Sunovion Pharmaceuticals, KCR, Janssen, Otsuka, Apodemus, Cortexyme, Acadia, MSD. WC has received grants from: Acadia, Alkermes, Allergan, Angelini, Auspex Pharmaceuticals, BMS, Celon, Cephalon, Cortexyme, Ferrier, Forest Laboratories, GedeonRichter, GWPharmaceuticals, HMNC Brain Health, IntraCellular Therapies, Janssen, KCR, Lilly, Lundbeck, Minerva, MSD, NIH, Novartis, Orion, Otsuka, Sanofi, Servier Honoraria: Adamed, Angelini, AstraZeneca, BMS, Celon, GSK, Janssen, KRKA, Lekam, Lundbeck, Minerva, NeuroCog, Novartis, Orion, Pfizer, Polfa Tarchomin, Sanofi, Servier, Zentiva Advisory boards: Angelini, Celon (terminated), Douglas Pharmaceuticals, Janssen, MSD, Novartis, Sanofi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators 2018 . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study. Lancet. (2017) 392:1789–858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Center for Behavioral Health Statistics and Quality . 2015 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; (2016).
Publication types
LinkOut - more resources
Full Text Sources

