Evolution of interventional endoscopic ultrasound
- PMID: 37398926
- PMCID: PMC10313421
- DOI: 10.1093/gastro/goad038
Evolution of interventional endoscopic ultrasound
Abstract
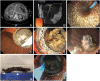
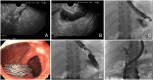
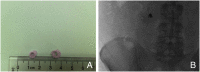
Endoscopic ultrasound (EUS) has become an indispensable modality for the assessment of the gastrointestinal tract and adjacent structures since its origin in the 1980s. Following the development of the linear echoendoscope, EUS has evolved from a purely diagnostic modality to a sophisticated tool for intervention, with numerous luminal, pancreaticobiliary, and hepatic applications. Broadly, these applications may be subdivided into three categories: transluminal drainage or access procedures, injection therapy, and EUS-guided liver interventions. Transluminal drainage or access procedures include management of pancreatic fluid collection, EUS-guided biliary drainage, EUS-guided bile duct drainage, EUS-guided pancreatic duct drainage, and enteral anastomosis formation. Injection therapies include therapeutic EUS-guided injections for management of malignancies accessible by EUS. EUS-guided liver applications include EUS-guided liver biopsy, EUS-guided portal pressure gradient measurement, and EUS-guided vascular therapies. In this review, we discuss the origins of each of these EUS applications, evolution of techniques leading to the current status, and future directions of EUS-guided interventional therapy.
Keywords: biliary drainage; endoscopic ultrasound; gastrojejunostomy; interventional endoscopic ultrasound; pancreatic pseudocyst drainage.
© The Author(s) 2023. Published by Oxford University Press and Sixth Affiliated Hospital of Sun Yat-sen University.
Conflict of interest statement
M.J.R. and D.S.S. have no conflicts of interest. V.M.S. is a consultant for Olympus America, Cook Medical, and Boston Scientific.
Figures



References
-
- Strohm WD, Phillip J, Hagenmüller F. et al. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy 1980;12:241–4. - PubMed
-
- Dimagno EP, Buxton JL, Regan PT. et al. Ultrasonic endoscope. Lancet 1980;1:629–31. - PubMed
-
- Rogers BH, Cicurel NJ, Seed RW.. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest Endosc 1975;21:133–4. - PubMed
-
- Kozarek RA, Brayko CM, Harlan J. et al. Endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc 1985;31:322–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

