Effectiveness of Nasal Continuous Positive Airway Pressure vs Nasal Intermittent Positive Pressure Ventilation vs Noninvasive High-Frequency Oscillatory Ventilation as Support After Extubation of Neonates Born Extremely Preterm or With More Severe Respiratory Failure: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 37399009
- PMCID: PMC10318479
- DOI: 10.1001/jamanetworkopen.2023.21644
Effectiveness of Nasal Continuous Positive Airway Pressure vs Nasal Intermittent Positive Pressure Ventilation vs Noninvasive High-Frequency Oscillatory Ventilation as Support After Extubation of Neonates Born Extremely Preterm or With More Severe Respiratory Failure: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: The NASONE (Nasal Oscillation Post-Extubation) trial showed that noninvasive high-frequency oscillatory ventilation (NHFOV) slightly reduces the duration of invasive mechanical ventilation (IMV) in preterm infants, whereas NHFOV and noninvasive intermittent positive pressure ventilation (NIPPV) result in fewer reintubations than nasal continuous positive airway pressure (NCPAP). It is unknown whether NHFOV is similarly effective in extremely preterm neonates or in those with more severe respiratory failure (based on the duration of previous ventilation and CO2 levels).
Objective: To clarify whether NHFOV is better than NIPPV and NCPAP in reducing the duration of IMV in extremely preterm neonates or those with severe respiratory failure.
Design, setting, and participants: This study is a predefined secondary analyses of a multicenter randomized clinical trial that was performed at tertiary academic neonatal intensive care units (NICUs) in China. Participants included neonates enrolled in the NASONE trial between December 2017 and May 2021 and belonging to 3 predefined subgroups: (1) born at less than or equal to 28 weeks' (plus 6 days) gestation, (2) invasively ventilated for more than 1 week from birth, and (3) with CO2 greater than 50 mm Hg before or in the 24 hours after extubation. Data analysis was performed in August 2022.
Intervention: NCPAP, NIPPV, or NHFOV since the first extubation and until NICU discharge, with airway pressure higher in NHFOV than in NIPPV and than in NCPAP.
Main outcomes and measures: The co-primary outcomes were total duration of IMV during the NICU stay, need for reintubation, and ventilator-free days calculated as per the original trial protocol. Outcomes were analyzed on an intention-to-treat basis as for the whole trial, and subgroup analyses followed the original statistical plan.
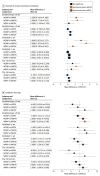
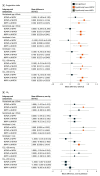
Results: Among 1137 preterm infants, 455 (279 boys [61.3%]) were born at 28 weeks' gestation or less, 375 (218 boys [58.1%]) underwent IMV for more than 1 week from birth, and 307 (183 boys [59.6%]) had CO2 greater than 50 mm Hg before or in the 24 hours after extubation. Both NIPPV and NHFOV were associated with significantly fewer reintubations (risk difference range, -28% [95% CI, -39% to -17%] to -15% [95% CI, -25% to -4%]; number needed to treat, 3-7 infants) and early reintubations (risk difference range, -24% [95% CI, -35% to -14%] to -20% [95% CI, -30% to -10%]) than NCPAP, and these reintubations were less frequently due to refractory hypoxemia. IMV was shorter in the NIPPV and NHFOV groups (mean difference range, -5.0 days [95% CI, -6.8 to -3.1 days] to -2.3 days [95% CI, -4.1 to -0.4 days]) than in the NCPAP group. Co-primary outcomes were not different between NIPPV and NHFOV; there was no significant interaction effect. Infants in the NHFOV group showed significantly less moderate-to-severe bronchopulmonary dysplasia than infants in the NCPAP group (range, -12% to -10%; number needed to treat, 8-9 infants) and better postextubation gas exchange in all subgroups. The 3 interventions were provided at different mean airway pressure and were equally safe.
Conclusions and relevance: The subgroup analyses of extremely preterm or more ill infants confirm the results obtained in the whole population: NIPPV and NHFOV appeared equally effective in reducing duration of IMV compared with NCPAP.
Trial registration: ClinicalTrials.gov Identifier: NCT03181958.
Conflict of interest statement
Figures


References
-
- Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015;169(11):1011-1017. doi:10.1001/jamapediatrics.2015.2401 - DOI - PMC - PubMed
-
- Shi Y, De Luca D; NASal OscillatioN post-Extubation (NASONE) Study Group . Continuous positive airway pressure (CPAP) vs noninvasive positive pressure ventilation (NIPPV) vs noninvasive high frequency oscillation ventilation (NHFOV) as post-extubation support in preterm neonates: protocol for an assessor-blinded, multicenter, randomized controlled trial. BMC Pediatr. 2019;19(1):256. doi:10.1186/s12887-019-1625-1 - DOI - PMC - PubMed

