Mapping Lesion-Related Epilepsy to a Human Brain Network
- PMID: 37399040
- PMCID: PMC10318550
- DOI: 10.1001/jamaneurol.2023.1988
Mapping Lesion-Related Epilepsy to a Human Brain Network
Abstract
Importance: It remains unclear why lesions in some locations cause epilepsy while others do not. Identifying the brain regions or networks associated with epilepsy by mapping these lesions could inform prognosis and guide interventions.
Objective: To assess whether lesion locations associated with epilepsy map to specific brain regions and networks.
Design, setting, and participants: This case-control study used lesion location and lesion network mapping to identify the brain regions and networks associated with epilepsy in a discovery data set of patients with poststroke epilepsy and control patients with stroke. Patients with stroke lesions and epilepsy (n = 76) or no epilepsy (n = 625) were included. Generalizability to other lesion types was assessed using 4 independent cohorts as validation data sets. The total numbers of patients across all datasets (both discovery and validation datasets) were 347 with epilepsy and 1126 without. Therapeutic relevance was assessed using deep brain stimulation sites that improve seizure control. Data were analyzed from September 2018 through December 2022. All shared patient data were analyzed and included; no patients were excluded.
Main outcomes and measures: Epilepsy or no epilepsy.
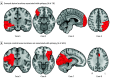
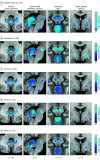
Results: Lesion locations from 76 patients with poststroke epilepsy (39 [51%] male; mean [SD] age, 61.0 [14.6] years; mean [SD] follow-up, 6.7 [2.0] years) and 625 control patients with stroke (366 [59%] male; mean [SD] age, 62.0 [14.1] years; follow-up range, 3-12 months) were included in the discovery data set. Lesions associated with epilepsy occurred in multiple heterogenous locations spanning different lobes and vascular territories. However, these same lesion locations were part of a specific brain network defined by functional connectivity to the basal ganglia and cerebellum. Findings were validated in 4 independent cohorts including 772 patients with brain lesions (271 [35%] with epilepsy; 515 [67%] male; median [IQR] age, 60 [50-70] years; follow-up range, 3-35 years). Lesion connectivity to this brain network was associated with increased risk of epilepsy after stroke (odds ratio [OR], 2.82; 95% CI, 2.02-4.10; P < .001) and across different lesion types (OR, 2.85; 95% CI, 2.23-3.69; P < .001). Deep brain stimulation site connectivity to this same network was associated with improved seizure control (r, 0.63; P < .001) in 30 patients with drug-resistant epilepsy (21 [70%] male; median [IQR] age, 39 [32-46] years; median [IQR] follow-up, 24 [16-30] months).
Conclusions and relevance: The findings in this study indicate that lesion-related epilepsy mapped to a human brain network, which could help identify patients at risk of epilepsy after a brain lesion and guide brain stimulation therapies.
Conflict of interest statement
Figures



Comment in
-
Reframing Lesional Epilepsy as a Network Disease.JAMA Neurol. 2023 Sep 1;80(9):889-890. doi: 10.1001/jamaneurol.2023.1080. JAMA Neurol. 2023. PMID: 37399027 No abstract available.
-
"Navigating the network": localising the lesion with the advent of lesion network mapping.J Neurol. 2023 Dec;270(12):6207-6209. doi: 10.1007/s00415-023-12059-5. Epub 2023 Oct 26. J Neurol. 2023. PMID: 37882826 Free PMC article. No abstract available.
-
Seizing the Brain Networks in Lesional Focal Epilepsies.Epilepsy Curr. 2023 Dec 12;24(1):28-30. doi: 10.1177/15357597231216917. eCollection 2024 Jan-Feb. Epilepsy Curr. 2023. PMID: 38327534 Free PMC article.
References
-
- Galovic M, Döhler N, Erdélyi-Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 2018;17(2):143-152. doi: 10.1016/S1474-4422(17)30404-0 - DOI - PubMed

