Insulin feedback is a targetable resistance mechanism of PI3K inhibition in glioblastoma
- PMID: 37399061
- PMCID: PMC10708938
- DOI: 10.1093/neuonc/noad117
Insulin feedback is a targetable resistance mechanism of PI3K inhibition in glioblastoma
Abstract
Background: Insulin feedback is a critical mechanism responsible for the poor clinical efficacy of phosphatidylinositol 3-kinase (PI3K) inhibition in cancer, and hyperglycemia is an independent factor associated with poor prognosis in glioblastoma (GBM). We investigated combination anti-hyperglycemic therapy in a mouse model of GBM and evaluated the association of glycemic control in clinical trial data from patients with GBM.
Methods: The effect of the anti-hyperglycemic regimens, metformin and the ketogenic diet, was evaluated in combination with PI3K inhibition in patient-derived GBM cells and in an orthotopic GBM mouse model. Insulin feedback and the immune microenvironment were retrospectively evaluated in blood and tumor tissue from a Phase 2 clinical trial of buparlisib in patients with recurrent GBM.
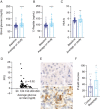
Results: We found that PI3K inhibition induces hyperglycemia and hyperinsulinemia in mice and that combining metformin with PI3K inhibition improves the treatment efficacy in an orthotopic GBM xenograft model. Through examination of clinical trial data, we found that hyperglycemia was an independent factor associated with poor progression-free survival in patients with GBM. We also found that PI3K inhibition increased insulin receptor activation and T-cell and microglia abundance in tumor tissue from these patients.
Conclusion: Reducing insulin feedback improves the efficacy of PI3K inhibition in GBM in mice, and hyperglycemia worsens progression-free survival in patients with GBM treated with PI3K inhibition. These findings indicate that hyperglycemia is a critical resistance mechanism associated with PI3K inhibition in GBM and that anti-hyperglycemic therapy may enhance PI3K inhibitor efficacy in GBM patients.
Keywords: glioblastoma; hyperglycemia; insulin; metformin; phosphatidylinositol 3-kinase.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2023.
Figures




References
-
- Goncalves MD, Hopkins BD, Cantley LC.. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379(21):2052–2062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

