Outer membrane translocation of pyocins via the copper regulated TonB-dependent transporter CrtA
- PMID: 37399084
- PMCID: PMC10422930
- DOI: 10.1042/BCJ20220552
Outer membrane translocation of pyocins via the copper regulated TonB-dependent transporter CrtA
Abstract
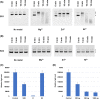
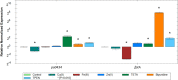
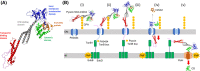
Pseudomonas aeruginosa is a common cause of serious hospital-acquired infections, the leading proven cause of mortality in people with cystic fibrosis and is associated with high levels of antimicrobial resistance. Pyocins are narrow-spectrum protein antibiotics produced by P. aeruginosa that kill strains of the same species and have the potential to be developed as therapeutics targeting multi-drug resistant isolates. We have identified two novel pyocins designated SX1 and SX2. Pyocin SX1 is a metal-dependent DNase while pyocin SX2 kills cells through inhibition of protein synthesis. Mapping the uptake pathways of SX1 and SX2 shows these pyocins utilize a combination of the common polysaccharide antigen (CPA) and a previously uncharacterized TonB-dependent transporter (TBDT) PA0434 to traverse the outer membrane. In addition, TonB1 and FtsH are required by both pyocins to energize their transport into cells and catalyze their translocation across the inner membrane, respectively. Expression of PA0434 was found to be specifically regulated by copper availability and we have designated PA0434 as Copper Responsive Transporter A, or CrtA. To our knowledge these are the first S-type pyocins described that utilize a TBDT that is not involved in iron uptake.
Keywords: Pseudomonas aeruginosa; antibiotic resistance; bacteriocin; pyocin.
© 2023 The Author(s).
Conflict of interest statement
D.W. and C.K. are co-founders of Glox Therapeutics and own shares in this company. Glox develops bacteriocin based antibiotics. D.W. and C.K. are also authors on patents relating to the use of bacteriocins in treating infection.
Figures




Similar articles
-
Pyocin S5 Import into Pseudomonas aeruginosa Reveals a Generic Mode of Bacteriocin Transport.mBio. 2020 Mar 10;11(2):e03230-19. doi: 10.1128/mBio.03230-19. mBio. 2020. PMID: 32156826 Free PMC article.
-
A Colicin M-Type Bacteriocin from Pseudomonas aeruginosa Targeting the HxuC Heme Receptor Requires a Novel Immunity Partner.Appl Environ Microbiol. 2018 Aug 31;84(18):e00716-18. doi: 10.1128/AEM.00716-18. Print 2018 Sep 15. Appl Environ Microbiol. 2018. PMID: 29980560 Free PMC article.
-
Targeted Killing of Pseudomonas aeruginosa by Pyocin G Occurs via the Hemin Transporter Hur.J Mol Biol. 2020 Jun 12;432(13):3869-3880. doi: 10.1016/j.jmb.2020.04.020. Epub 2020 Apr 25. J Mol Biol. 2020. PMID: 32339530 Free PMC article.
-
Pyocins and Beyond: Exploring the World of Bacteriocins in Pseudomonas aeruginosa.Probiotics Antimicrob Proteins. 2025 Feb;17(1):240-252. doi: 10.1007/s12602-024-10322-3. Epub 2024 Jul 18. Probiotics Antimicrob Proteins. 2025. PMID: 39023701 Review.
-
The pyocins of Pseudomonas aeruginosa.Biochimie. 2002 May-Jun;84(5-6):499-510. doi: 10.1016/s0300-9084(02)01422-0. Biochimie. 2002. PMID: 12423794 Review.
Cited by
-
A pyocin-like T6SS effector mediates bacterial competition in Yersinia pseudotuberculosis.Microbiol Spectr. 2024 Jun 4;12(6):e0427823. doi: 10.1128/spectrum.04278-23. Epub 2024 May 7. Microbiol Spectr. 2024. PMID: 38712967 Free PMC article.
-
Structural constraints of pyocin S2 import through the ferripyoverdine receptor FpvAI.PNAS Nexus. 2024 Mar 27;3(4):pgae124. doi: 10.1093/pnasnexus/pgae124. eCollection 2024 Apr. PNAS Nexus. 2024. PMID: 38577260 Free PMC article.
References
-
- WHO. (2017) Prioritization of Pathogens to Guide Discovery, Research and Development of new Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis, WHO, Geneva, Switzerland
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

