A Comparative Study of Ultrasmall Calcium Carbonate Nanoparticles for Targeting and Imaging Atherosclerotic Plaque
- PMID: 37399106
- PMCID: PMC10900527
- DOI: 10.1021/acsnano.3c03523
A Comparative Study of Ultrasmall Calcium Carbonate Nanoparticles for Targeting and Imaging Atherosclerotic Plaque
Abstract
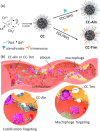
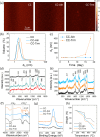
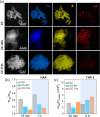
Atherosclerosis is a complex disease that can lead to life-threatening events, such as myocardial infarction and ischemic stroke. Despite the severity of this disease, diagnosing plaque vulnerability remains challenging due to the lack of effective diagnostic tools. Conventional diagnostic protocols lack specificity and fail to predict the type of atherosclerotic lesion and the risk of plaque rupture. To address this issue, technologies are emerging, such as noninvasive medical imaging of atherosclerotic plaque with customized nanotechnological solutions. Modulating the biological interactions and contrast of nanoparticles in various imaging techniques, including magnetic resonance imaging, is possible through the careful design of their physicochemical properties. However, few examples of comparative studies between nanoparticles targeting different hallmarks of atherosclerosis exist to provide information about the plaque development stage. Our work demonstrates that Gd (III)-doped amorphous calcium carbonate nanoparticles are an effective tool for these comparative studies due to their high magnetic resonance contrast and physicochemical properties. In an animal model of atherosclerosis, we compare the imaging performance of three types of nanoparticles: bare amorphous calcium carbonate and those functionalized with the ligands alendronate (for microcalcification targeting) and trimannose (for inflammation targeting). Our study provides useful insights into ligand-mediated targeted imaging of atherosclerosis through a combination of in vivo imaging, ex vivo tissue analysis, and in vitro targeting experiments.
Keywords: amorphous calcium carbonate nanoparticles; atherosclerosis; ligand-mediated targeted imaging; magnetic resonance imaging; synchrotron X-ray fluorescence tissue analysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Vigne J.; Thackeray J.; Essers J.; Makowski M.; Varasteh Z.; Curaj A.; Karlas A.; Canet-Soulas E.; Mulder W.; Kiessling F.; Schäfers M.; Botnar R.; Wildgruber M.; Hyafil F. Cardiovascular study group of the European Society of Molecular, Current and Emerging Preclinical Approaches for Imaging-Based Characterization of Atherosclerosis. Mol. Imaging Biol. 2018, 20, 869–887. 10.1007/s11307-018-1264-1. - DOI - PubMed

