Subspace partitioning in the human prefrontal cortex resolves cognitive interference
- PMID: 37399398
- PMCID: PMC10334727
- DOI: 10.1073/pnas.2220523120
Subspace partitioning in the human prefrontal cortex resolves cognitive interference
Abstract
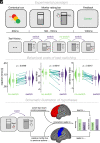
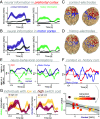
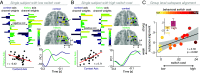
The human prefrontal cortex (PFC) constitutes the structural basis underlying flexible cognitive control, where mixed-selective neural populations encode multiple task features to guide subsequent behavior. The mechanisms by which the brain simultaneously encodes multiple task-relevant variables while minimizing interference from task-irrelevant features remain unknown. Leveraging intracranial recordings from the human PFC, we first demonstrate that competition between coexisting representations of past and present task variables incurs a behavioral switch cost. Our results reveal that this interference between past and present states in the PFC is resolved through coding partitioning into distinct low-dimensional neural states; thereby strongly attenuating behavioral switch costs. In sum, these findings uncover a fundamental coding mechanism that constitutes a central building block of flexible cognitive control.
Keywords: cognitive control; intracranial EEG; population geometry; prefrontal cortex.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Parthasarathy A., et al. , Mixed selectivity morphs population codes in prefrontal cortex. Nat. Neurosci. 20, 1770–1779 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

