RNF213 loss-of-function promotes pathological angiogenesis in moyamoya disease via the Hippo pathway
- PMID: 37399508
- PMCID: PMC10629795
- DOI: 10.1093/brain/awad225
RNF213 loss-of-function promotes pathological angiogenesis in moyamoya disease via the Hippo pathway
Abstract
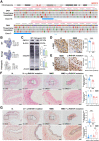
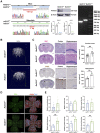
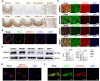
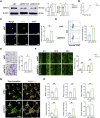
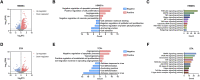
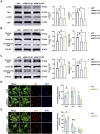
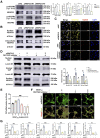
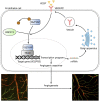
Moyamoya disease is an uncommon cerebrovascular disorder characterized by steno-occlusive changes in the circle of Willis and abnormal vascular network development. Ring finger protein 213 (RNF213) has been identified as an important susceptibility gene for Asian patients, but researchers have not completely elucidated whether RNF213 mutations affect the pathogenesis of moyamoya disease. Using donor superficial temporal artery samples, whole-genome sequencing was performed to identify RNF213 mutation types in patients with moyamoya disease, and histopathology was performed to compare morphological differences between patients with moyamoya disease and intracranial aneurysm. The vascular phenotype of RNF213-deficient mice and zebrafish was explored in vivo, and RNF213 knockdown in human brain microvascular endothelial cells was employed to analyse cell proliferation, migration and tube formation abilities in vitro. After bioinformatics analysis of both cell and bulk RNA-seq data, potential signalling pathways were measured in RNF213-knockdown or RNF213-knockout endothelial cells. We found that patients with moyamoya disease carried pathogenic mutations of RNF213 that were positively associated with moyamoya disease histopathology. RNF213 deletion exacerbated pathological angiogenesis in the cortex and retina. Reduced RNF213 expression led to increased endothelial cell proliferation, migration and tube formation. Endothelial knockdown of RNF213 activated the Hippo pathway effector Yes-associated protein (YAP)/tafazzin (TAZ) and promoted the overexpression of the downstream effector VEGFR2. Additionally, inhibition of YAP/TAZ resulted in altered cellular VEGFR2 distribution due to defects in trafficking from the Golgi apparatus to the plasma membrane and reversed RNF213 knockdown-induced angiogenesis. All these key molecules were validated in ECs isolated from RNF213-deficient animals. Our findings may suggest that loss-of-function of RNF213 mediates the pathogenesis of moyamoya disease via the Hippo pathway.
Keywords: RNF213; angiogenesis; hippo pathway; moyamoya disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures
References
-
- Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226–1237. - PubMed
-
- Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008;79:900–904. - PubMed
-
- Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for moyamoya disease. Neurol Med Chir (Tokyo). 2007;47:1–4. - PubMed
-
- Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases . Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of willis). Neurol Med Chir (Tokyo). 2012;52:245–266. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

