C1 inhibitor and prolylcarboxypeptidase modulate prekallikrein activation on endothelial cells
- PMID: 37399947
- PMCID: PMC10592223
- DOI: 10.1016/j.jaci.2023.06.017
C1 inhibitor and prolylcarboxypeptidase modulate prekallikrein activation on endothelial cells
Abstract
Background: We examined how prekallikrein (PK) activation on human microvascular endothelial cells (HMVECs) is regulated by the ambient concentration of C1 inhibitor (C1INH) and prolylcarboxypeptidase (PRCP).
Objective: We sought to examine the specificity of PK activation on HMVECs by PRCP and the role of C1INH to regulate it, high-molecular-weight kininogen (HK) cleavage, and bradykinin (BK) liberation.
Methods: Investigations were performed on cultured HMVECs. Immunofluorescence, enzymatic activity assays, immunoblots, small interfering RNA knockdowns, and cell transfections were used to perform these studies.
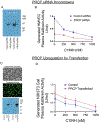
Results: Cultured HMVECs constitutively coexpressed PK, HK, C1INH, and PRCP. PK activation on HMVECs was modulated by the ambient C1INH concentration. In the absence of C1INH, forming PKa on HMVECs cleaved 120-kDa HK completely to a 65-kDa H-chain and a 46-kDa L-chain in 60 minutes. In the presence of 2 μM C1INH, only 50% of the HK became cleaved. C1INH concentrations (0.0-2.5 μM) decreased but did not abolish BK liberated from HK by activated PK. Factor XII did not activate when incubated with HMVECs alone for 1 hour. However, if incubated in the presence of HK and PK, factor XII became activated. The specificity of PK activation on HMVECs by PRCP was shown by several inhibitors to each enzyme. Furthermore, PRCP small interfering RNA knockdowns magnified C1INH inhibitory activity on PK activation, and PRCP transfections reduced C1INH inhibition at any given concentration.
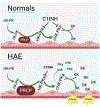
Conclusions: These combined studies indicated that on HMVECs, PK activation and HK cleavage to liberate BK were modulated by the local concentrations of C1INH and PRCP.
Keywords: C1 inhibitor; Prekallikrein; bradykinin; high-molecular-weight kininogen; plasma kallikrein; prolylcarboxypeptidase.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


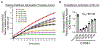


References
-
- Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016; 14:28–39. - PubMed
-
- Wiggins RC. Kinin release from high molecular weight kininogen by the action of Hageman factor in the absence of kallikrein. J Biol Chem. 1983;258:8963–70. - PubMed
-
- Girey GJ, Talamo RC, Colman RW. The kinetics of the release of bradykinin by kallikrein in normal human plasma. J Lab Clin Med. 1972;80:496–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

