Canine models of Charcot-Marie-Tooth: MTMR2, MPZ, and SH3TC2 variants in golden retrievers with congenital hypomyelinating polyneuropathy
- PMID: 37400349
- PMCID: PMC10530471
- DOI: 10.1016/j.nmd.2023.06.007
Canine models of Charcot-Marie-Tooth: MTMR2, MPZ, and SH3TC2 variants in golden retrievers with congenital hypomyelinating polyneuropathy
Abstract
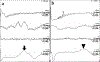
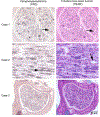
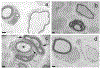
Congenital hypomyelinating polyneuropathy (HPN) restricted to the peripheral nervous system was reported in 1989 in two Golden Retriever (GR) littermates. Recently, four additional cases of congenital HPN in young, unrelated GRs were diagnosed via neurological examination, electrodiagnostic evaluation, and peripheral nerve pathology. Whole-genome sequencing was performed on all four GRs, and variants from each dog were compared to variants found across >1,000 other dogs, all presumably unaffected with HPN. Likely causative variants were identified for each HPN-affected GR. Two cases shared a homozygous splice donor site variant in MTMR2, with a stop codon introduced within six codons following the inclusion of the intron. One case had a heterozygous MPZ isoleucine to threonine substitution. The last case had a homozygous SH3TC2 nonsense variant predicted to truncate approximately one-half of the protein. Haplotype analysis using 524 GR established the novelty of the identified variants. Each variant occurs within genes that are associated with the human Charcot-Marie-Tooth (CMT) group of heterogeneous diseases, affecting the peripheral nervous system. Testing a large GR population (n = >200) did not identify any dogs with these variants. Although these variants are rare within the general GR population, breeders should be cautious to avoid propagating these alleles.
Keywords: Animal model; Dysmyelination; Electrodiagnostic testing; Genetic; Genocopies; Histopathology.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None for any authors or co-authors on “Canine models of Charcot-Marie-Tooth: MTMR2, MPZ, and SH3TC2 variants in Golden Retrievers with congenital hypomyelinating polyneuropathy” by Cook, et al. declare any conflicts of interest.
Figures