CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results
- PMID: 37400640
- PMCID: PMC11462637
- DOI: 10.1038/s41591-023-02404-6
CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results
Abstract
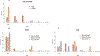
In preclinical models, anakinra, an IL-1 receptor antagonist (IL-1Ra), reduced immune effector cell-associated neurotoxicity syndrome (ICANS) without compromising anti-CD19 chimeric antigen receptor (CAR) T-cell efficacy. We initiated a phase 2 clinical trial of anakinra in patients with relapsed/refractory large B-cell lymphoma and mantle cell lymphoma treated with commercial anti-CD19 CAR T-cell therapy. Here we report a non-prespecified interim analysis reporting the final results from cohort 1 in which patients received subcutaneous anakinra from day 2 until at least day 10 post-CAR T-cell infusion. The primary endpoint was the rate of severe (grade ≥3) ICANS. Key secondary endpoints included the rates of all-grade cytokine release syndrome (CRS) and ICANS and overall disease response. Among 31 treated patients, 74% received axicabtagene ciloleucel, 13% received brexucabtagene ciloleucel and 4% received tisagenlecleucel. All-grade ICANS occurred in 19%, and severe ICANS occurred in 9.7% of patients. There were no grade 4 or 5 ICANS events. All-grade CRS occurred in 74%, and severe CRS occurred in 6.4% of patients. The overall disease response rate was 77% with 65% complete response rate. These initial results show that prophylactic anakinra resulted in a low incidence of ICANS in patients with lymphoma receiving anti-CD19 CAR T-cell therapy and support further study of anakinra in immune-related neurotoxicity syndromes.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests
Figures
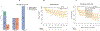
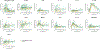

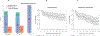

References
-
- Abramson JS, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (London, England) 396, 839–852 (2020). - PubMed
-
- Schuster SJ, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. The Lancet. Oncology 22, 1403–1415 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

