NNAT is a novel mediator of oxidative stress that suppresses ER + breast cancer
- PMID: 37400769
- PMCID: PMC10318825
- DOI: 10.1186/s10020-023-00673-y
NNAT is a novel mediator of oxidative stress that suppresses ER + breast cancer
Abstract
Background: Neuronatin (NNAT) was recently identified as a novel mediator of estrogen receptor-positive (ER+) breast cancer cell proliferation and migration, which correlated with decreased tumorigenic potential and prolonged patient survival. However, despite these observations, the molecular and pathophysiological role(s) of NNAT in ER + breast cancer remains unclear. Based on high protein homology with phospholamban, we hypothesized that NNAT mediates the homeostasis of intracellular calcium [Ca2+]i levels and endoplasmic reticulum (EndoR) function, which is frequently disrupted in ER + breast cancer and other malignancies.
Methods: To evaluate the role of NNAT on [Ca2+]i homeostasis, we used a combination of bioinformatics, gene expression and promoter activity assays, CRISPR gene manipulation, pharmacological tools and confocal imaging to characterize the association between ROS, NNAT and calcium signaling.
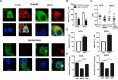
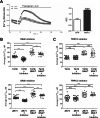
Results: Our data indicate that NNAT localizes predominantly to EndoR and lysosome, and genetic manipulation of NNAT levels demonstrated that NNAT modulates [Ca2+]i influx and maintains Ca2+ homeostasis. Pharmacological inhibition of calcium channels revealed that NNAT regulates [Ca2+]i levels in breast cancer cells through the interaction with ORAI but not the TRPC signaling cascade. Furthermore, NNAT is transcriptionally regulated by NRF1, PPARα, and PPARγ and is strongly upregulated by oxidative stress via the ROS and PPAR signaling cascades.
Conclusion: Collectively, these data suggest that NNAT expression is mediated by oxidative stress and acts as a regulator of Ca2+ homeostasis to impact ER + breast cancer proliferation, thus providing a molecular link between the longstanding observation that is accumulating ROS and altered Ca2+ signaling are key oncogenic drivers of cancer.
Keywords: Breast cancer; Neuronatin; Orai; PPAR; ROS.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

