Characteristics of glucose and lipid metabolism and the interaction between gut microbiota and colonic mucosal immunity in pigs during cold exposure
- PMID: 37400906
- PMCID: PMC10318708
- DOI: 10.1186/s40104-023-00886-5
Characteristics of glucose and lipid metabolism and the interaction between gut microbiota and colonic mucosal immunity in pigs during cold exposure
Abstract
Background: Cold regions have long autumn and winter seasons and low ambient temperatures. When pigs are unable to adjust to the cold, oxidative damage and inflammation may develop. However, the differences between cold and non-cold adaptation regarding glucose and lipid metabolism, gut microbiota and colonic mucosal immunological features in pigs are unknown. This study revealed the glucose and lipid metabolic responses and the dual role of gut microbiota in pigs during cold and non-cold adaptation. Moreover, the regulatory effects of dietary glucose supplements on glucose and lipid metabolism and the colonic mucosal barrier were evaluated in cold-exposed pigs.
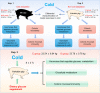
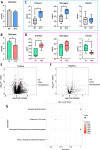
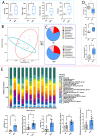
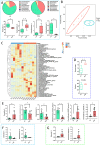
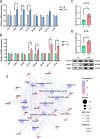
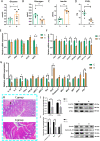
Results: Cold and non-cold-adapted models were established by Min and Yorkshire pigs. Our results exhibited that cold exposure induced glucose overconsumption in non-cold-adapted pig models (Yorkshire pigs), decreasing plasma glucose concentrations. In this case, cold exposure enhanced the ATGL and CPT-1α expression to promote liver lipolysis and fatty acid oxidation. Meanwhile, the two probiotics (Collinsella and Bifidobacterium) depletion and the enrichment of two pathogens (Sutterella and Escherichia-Shigella) in colonic microbiota are not conducive to colonic mucosal immunity. However, glucagon-mediated hepatic glycogenolysis in cold-adapted pig models (Min pigs) maintained the stability of glucose homeostasis during cold exposure. It contributed to the gut microbiota (including the enrichment of the Rikenellaceae RC9 gut group, [Eubacterium] coprostanoligenes group and WCHB1-41) that favored cold-adapted metabolism.
Conclusions: The results of both models indicate that the gut microbiota during cold adaptation contributes to the protection of the colonic mucosa. During non-cold adaptation, cold-induced glucose overconsumption promotes thermogenesis through lipolysis, but interferes with the gut microbiome and colonic mucosal immunity. Furthermore, glucagon-mediated hepatic glycogenolysis contributes to glucose homeostasis during cold exposure.
Keywords: Cold exposure; Colonic mucosal immunity; Fatty acid oxidation; Glucose and lipid metabolism; Gut microbiota; Pig model.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Glucose supplementation improves intestinal amino acid transport and muscle amino acid pool in pigs during chronic cold exposure.Anim Nutr. 2022 Dec 9;12:360-374. doi: 10.1016/j.aninu.2022.10.009. eCollection 2023 Mar. Anim Nutr. 2022. PMID: 36788930 Free PMC article.
-
Dietary-fat supplementation alleviates cold temperature-induced metabolic dysbiosis and barrier impairment by remodeling gut microbiota.Food Funct. 2024 Feb 5;15(3):1443-1459. doi: 10.1039/d3fo04916g. Food Funct. 2024. PMID: 38226701
-
Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs.Microbiome. 2022 Jul 30;10(1):115. doi: 10.1186/s40168-022-01303-1. Microbiome. 2022. PMID: 35907917 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics.Benef Microbes. 2014 Mar;5(1):3-17. doi: 10.3920/BM2012.0065. Benef Microbes. 2014. PMID: 23886976 Review.
Cited by
-
Maternal supplementation with konjac glucomannan and κ-carrageenan promotes sow performance and benefits the gut barrier in offspring.Anim Nutr. 2024 Sep 5;19:272-286. doi: 10.1016/j.aninu.2024.05.011. eCollection 2024 Dec. Anim Nutr. 2024. PMID: 39640556 Free PMC article.
-
Composite Probiotics Improve Gut Health and Enhance Tryptophan Metabolism in Nursery Piglets During Liquid Feeding.Int J Mol Sci. 2025 Jun 13;26(12):5698. doi: 10.3390/ijms26125698. Int J Mol Sci. 2025. PMID: 40565160 Free PMC article.
-
Accurate models and nutritional strategies for specific oxidative stress factors: Does the dose matter in swine production?J Anim Sci Biotechnol. 2024 Jan 26;15(1):11. doi: 10.1186/s40104-023-00964-8. J Anim Sci Biotechnol. 2024. PMID: 38273345 Free PMC article. Review.
-
A new evaluation system for drug-microbiota interactions.Imeta. 2024 May 7;3(3):e199. doi: 10.1002/imt2.199. eCollection 2024 Jun. Imeta. 2024. PMID: 38898986 Free PMC article.
-
Alkaline Mineral Complex Water Attenuates Transportation-Induced Hepatic Lipid Metabolism Dysregulation by AMPKα-SREBP-1c/PPARα Pathways.Int J Mol Sci. 2024 Oct 23;25(21):11373. doi: 10.3390/ijms252111373. Int J Mol Sci. 2024. PMID: 39518926 Free PMC article.
References
-
- Toghiani S, Hay E, Roberts A, Rekaya R. Impact of cold stress on birth and weaning weight in a composite beef cattle breed. Livest Sci. 2020;236:104053. doi: 10.1016/j.livsci.2020.104053. - DOI
-
- da Rosa G, Dazuk V, Alba DF, Galli GM, Molosse V, Boiago MM, et al. Curcumin addition in diet of laying hens under cold stress has antioxidant and antimicrobial effects and improves bird health and egg quality. J Therm Biol. 2020;91:102618–27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

