Aged mice are less susceptible to motion sickness and show decreased efferent vestibular activity compared to young adults
- PMID: 37401009
- PMCID: PMC10454360
- DOI: 10.1002/brb3.3064
Aged mice are less susceptible to motion sickness and show decreased efferent vestibular activity compared to young adults
Abstract
Introduction: The efferent vestibular system (EVS) is a feedback circuit thought to modulate vestibular afferent activity by inhibiting type II hair cells and exciting calyx-bearing afferents in the peripheral vestibular organs. In a previous study, we suggested EVS activity may contribute to the effects of motion sickness. To determine an association between motion sickness and EVS activity, we examined the effects of provocative motion (PM) on c-Fos expression in brainstem efferent vestibular nucleus (EVN) neurons that are the source of efferent innervation in the peripheral vestibular organs.
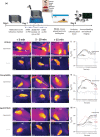
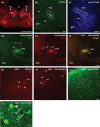
Methods: c-Fos is an immediate early gene product expressed in stimulated neurons and is a well-established marker of neuronal activation. To study the effects of PM, young adult C57/BL6 wild-type (WT), aged WT, and young adult transgenic Chat-gCaMP6f mice were exposed to PM, and tail temperature (Ttail ) was monitored using infrared imaging. After PM, we used immunohistochemistry to label EVN neurons to determine any changes in c-Fos expression. All tissue was imaged using laser scanning confocal microscopy.
Results: Infrared recording of Ttail during PM indicated that young adult WT and transgenic mice displayed a typical motion sickness response (tail warming), but not in aged WT mice. Similarly, brainstem EVN neurons showed increased expression of c-Fos protein after PM in young adult WT and transgenic mice but not in aged cohorts.
Conclusion: We present evidence that motion sickness symptoms and increased activation of EVN neurons occur in young adult WT and transgenic mice in response to PM. In contrast, aged WT mice showed no signs of motion sickness and no change in c-Fos expression when exposed to the same provocative stimulus.
Keywords: aging; c-Fos; motion sickness; vestibular efferent.
© 2023 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Agrawal, Y. , Merfeld, D. M. , Horak, F. B. , Redfern, M. S. , Manor, B. , Westlake, K. P. , Holstein, G. R. , Smith, P. F. , Bhatt, T. , Bohnen, N. I. , & Lipsitz, L. A. (2020). Aging, vestibular function, and balance: Proceedings of a national institute on aging/national institute on deafness and other communication disorders workshop. The Journals of Gerontology: Series A, 75(12), 2471–2480. - PMC - PubMed
-
- Benchaoui, H. , Siedek, E. , De La Puente‐Redondo, V. , Tilt, N. , Rowan, T. , & Clemence, R. (2007). Efficacy of maropitant for preventing vomiting associated with motion sickness in dogs. Veterinary Record, 161(13), 444–447. - PubMed
-
- Bergström, B. (1973a). Morphology of the vestibular nerve: II. The number of myelinated vestibular nerve fibers in man at various ages. Acta Oto‐laryngologica, 76(1–6), 173–179. - PubMed
-
- Bergström, B. (1973b). Morphology of the vestibular nerve: I. Anatomical studies of the vestibular nerve in man. Acta Oto‐Laryngologica, 76(1‐6), 162–172. - PubMed
-
- Borison, H. L. (1989). Area postrema: Chemoreceptor circumventricular organ of the medulla oblongata. Progress in Neurobiology, 32(5), 351–390. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

