Association of the CHEK2 c.1100delC variant, radiotherapy, and systemic treatment with contralateral breast cancer risk and breast cancer-specific survival
- PMID: 37401034
- PMCID: PMC10469654
- DOI: 10.1002/cam4.6272
Association of the CHEK2 c.1100delC variant, radiotherapy, and systemic treatment with contralateral breast cancer risk and breast cancer-specific survival
Abstract
Background: Breast cancer (BC) patients with a germline CHEK2 c.1100delC variant have an increased risk of contralateral BC (CBC) and worse BC-specific survival (BCSS) compared to non-carriers.
Aim: To assessed the associations of CHEK2 c.1100delC, radiotherapy, and systemic treatment with CBC risk and BCSS.
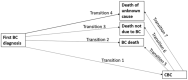
Methods: Analyses were based on 82,701 women diagnosed with a first primary invasive BC including 963 CHEK2 c.1100delC carriers; median follow-up was 9.1 years. Differential associations with treatment by CHEK2 c.1100delC status were tested by including interaction terms in a multivariable Cox regression model. A multi-state model was used for further insight into the relation between CHEK2 c.1100delC status, treatment, CBC risk and death.
Results: There was no evidence for differential associations of therapy with CBC risk by CHEK2 c.1100delC status. The strongest association with reduced CBC risk was observed for the combination of chemotherapy and endocrine therapy [HR (95% CI): 0.66 (0.55-0.78)]. No association was observed with radiotherapy. Results from the multi-state model showed shorter BCSS for CHEK2 c.1100delC carriers versus non-carriers also after accounting for CBC occurrence [HR (95% CI): 1.30 (1.09-1.56)].
Conclusion: Systemic therapy was associated with reduced CBC risk irrespective of CHEK2 c.1100delC status. Moreover, CHEK2 c.1100delC carriers had shorter BCSS, which appears not to be fully explained by their CBC risk.
Keywords: CHEK2 c.1100delC germline genetic variant; contralateral breast cancer risk; radiotherapy; survival; systemic treatment.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Matthias W. Beckmann and Peter A. Fasching conduct research funded by Amgen, Novartis and Pfizer (not related to this study). Peter A. Fasching received Honoraria from Roche, Novartis and Pfizer (not related to this study). Allison W. Kurian's institution received a research funding from Myriad genetics for an unrelated project (not related to this study). Emmanouil Saloustros reported the following: honoraria from Amgen Hellas, Pfizer Hellas, and IPSEN (not related to this study) and support from Merck Greece and Pfizer Hellas for attending meetings (not related to this study); he participated in advisory boards in Greece (MSD Greece, AstraZeneca Greece, Gilead Sciences Hellas, Pfizer Hellas, Genesis Pharma), not related to this study; he is a PI in sponsored clinical trials: MSD Greece (not related to this study). The other authors declare no conflict of interest.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA058860/CA/NCI NIH HHS/United States
- HHSN261201800015I/CA/NCI NIH HHS/United States
- G1000143/MRC_/Medical Research Council/United Kingdom
- R01 CA192393/CA/NCI NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
- UM1 CA186107/CA/NCI NIH HHS/United States
- U01 CA098758/CA/NCI NIH HHS/United States
- 25514/CRUK_/Cancer Research UK/United Kingdom
- R01 CA063464/CA/NCI NIH HHS/United States
- MR/M012190/1/MRC_/Medical Research Council/United Kingdom
- UM1 CA164917/CA/NCI NIH HHS/United States
- R01 CA163353/CA/NCI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- HHSN261201800032I/CA/NCI NIH HHS/United States
- NU58DP006320/CC/CDC HHS/United States
- R01 CA047147/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- R01 CA132839/CA/NCI NIH HHS/United States
- R01 CA177150/CA/NCI NIH HHS/United States
- R01 CA097396/CA/NCI NIH HHS/United States
- R01 CA069664/CA/NCI NIH HHS/United States
- HHSN261201800016I/CA/NCI NIH HHS/United States
- HHSN261201800009I/CA/NCI NIH HHS/United States
- U01 CA176726/CA/NCI NIH HHS/United States
- R01 CA054281/CA/NCI NIH HHS/United States
- Z01 CP010119/ImNIH/Intramural NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA128978/CA/NCI NIH HHS/United States
- NU58DP006344/DP/NCCDPHP CDC HHS/United States
- U19 CA148537/CA/NCI NIH HHS/United States
- U01 CA164973/CA/NCI NIH HHS/United States
- P50 CA116201/CA/NCI NIH HHS/United States
- Z01 ES049030/ImNIH/Intramural NIH HHS/United States
- R01 CA176785/CA/NCI NIH HHS/United States
- R01 CA047305/CA/NCI NIH HHS/United States
- K07 CA092044/CA/NCI NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- U54 CA156733/CA/NCI NIH HHS/United States
- R35 CA253187/CA/NCI NIH HHS/United States
- R01 CA077398/CA/NCI NIH HHS/United States
- U19 CA148112/CA/NCI NIH HHS/United States
- U19 CA148065/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 CA164920/CA/NCI NIH HHS/United States
- UM1 CA176726/CA/NCI NIH HHS/United States
- U01 CA199277/CA/NCI NIH HHS/United States
- UM1 CA164920/CA/NCI NIH HHS/United States
- U01 CA179715/CA/NCI NIH HHS/United States
- R01 CA140286/CA/NCI NIH HHS/United States
- R01 CA116167/CA/NCI NIH HHS/United States
- R01 CA128931/CA/NCI NIH HHS/United States