Ultrasound reduces inflammation by modulating M1/M2 polarization of microglia through STAT1/STAT6/PPARγ signaling pathways
- PMID: 37401041
- PMCID: PMC10651950
- DOI: 10.1111/cns.14333
Ultrasound reduces inflammation by modulating M1/M2 polarization of microglia through STAT1/STAT6/PPARγ signaling pathways
Abstract
Introduction: Activated microglia can be polarized to the pro-inflammatory M1 phenotype and the anti-inflammatory M2 phenotype. Low-intensity pulsed ultrasound (LIPUS) can attenuate pro-inflammatory responses in activated microglia.
Objective: This study aimed to investigate the effects of LIPUS on M1/M2 polarization of microglial cells and the regulatory mechanisms associated with signaling pathways.
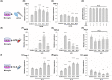
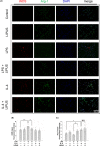
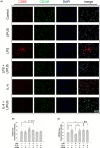
Methods: BV-2 microglial cells were stimulated by lipopolysaccharide (LPS) to an M1 phenotype or by interleukin-4 (IL-4) to an M2 phenotype. Some microglial cells were exposed to LIPUS, while others were not. M1/M2 marker mRNA and protein expression were measured using real-time polymerase chain reaction and western blot, respectively. Immunofluorescence staining was performed to determine inducible nitric oxide synthase (iNOS)-/arginase-1 (Arg-1)- and CD68-/CD206-positive cells.
Results: LIPUS treatment significantly attenuated LPS-induced increases in inflammatory markers (iNOS, tumor necrosis factor-α, interleukin-1β, and interleukin-6) as well as the expression of cell surface markers (CD86 and CD68) of M1-polarized microglia. In contrast, LIPUS treatment significantly enhanced the expression of M2-related markers (Arg-1, IL-10, and Ym1) and membrane protein (CD206). LIPUS treatment prevented M1 polarization of microglia and enhanced or sustained M2 polarization by regulating M1/M2 polarization through the signal transducer and activator of transcription 1/STAT6/peroxisome proliferator-activated receptor gamma pathways.
Conclusions: Our findings suggest that LIPUS inhibits microglial polarization and switches microglia from the M1 to the M2 phenotype.
Keywords: M1/M2; PPARγ; STAT; microglia polarization; neuroinflammation; ultrasound.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

