Hyaluronic acid and proteoglycan link protein 1 suppresses platelet‑derived growth factor-BB-induced proliferation, migration, and phenotypic switching of vascular smooth muscle cells
- PMID: 37401239
- PMCID: PMC10471460
- DOI: 10.5483/BMBRep.2023-0088
Hyaluronic acid and proteoglycan link protein 1 suppresses platelet‑derived growth factor-BB-induced proliferation, migration, and phenotypic switching of vascular smooth muscle cells
Abstract
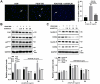
The development of atherosclerotic cardiovascular disease is associated with the phenotypic switching of vascular smooth muscle cells (SMCs) from a contractile to a synthetic state, leading to cell migration and proliferation. Platelet‑derived growth factor‑BB (PDGF‑BB) modulates this de-differentiation by initiating a number of biological processes. In this study, we show that gene expression of hyaluronic acid (HA) and proteoglycan link protein 1 (HAPLN1) was upregulated during differentiation of human aortic SMCs (HASMCs) into a contractile state, but downregulated upon during PDGF-BB-induced dedifferentiation. This is the first study showing that the treatment of HASMCs with full-length recombinant human HAPLN1 (rhHAPLN1) significantly reversed PDGF-BB-induced decrease in the protein levels of contractile markers (SM22α, α-SMA, calponin, and SM-MHC), and inhibited the proliferation and migration of HASMCs induced by PDGF-BB. Furthermore, our results show that rhHAPLN1 significantly inhibited the phosphorylation of FAK, AKT, STAT3, p38 MAPK and Raf mediated by the binding of PDGF-BB to PDGFRβ. Together, these results indicated that rhHAPLN1 can suppress the PDGF-BB-stimulated phenotypic switching and subsequent de-differentiation of HASMCs, highlighting its potential as a novel therapeutic target for atherosclerosis and other vascular diseases. [BMB Reports 2023; 56(8): 445-450].
Conflict of interest statement
D.Z., Z.F., J.M.J., G.Y. and I.C.S. are employees of HaplnScience Inc. J.M.J. and D.K.K. are shareholders of HaplnScience Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

