Oxidative stress induces lysosomal membrane permeabilization and ceramide accumulation in retinal pigment epithelial cells
- PMID: 37401371
- PMCID: PMC10399446
- DOI: 10.1242/dmm.050066
Oxidative stress induces lysosomal membrane permeabilization and ceramide accumulation in retinal pigment epithelial cells
Abstract
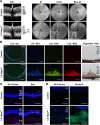
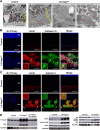
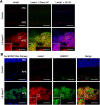
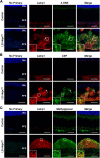
Oxidative stress has been implicated in the pathogenesis of age-related macular degeneration, the leading cause of blindness in older adults, with retinal pigment epithelium (RPE) cells playing a key role. To better understand the cytotoxic mechanisms underlying oxidative stress, we used cell culture and mouse models of iron overload, as iron can catalyze reactive oxygen species formation in the RPE. Iron-loading of cultured induced pluripotent stem cell-derived RPE cells increased lysosomal abundance, impaired proteolysis and reduced the activity of a subset of lysosomal enzymes, including lysosomal acid lipase (LIPA) and acid sphingomyelinase (SMPD1). In a liver-specific Hepc (Hamp) knockout murine model of systemic iron overload, RPE cells accumulated lipid peroxidation adducts and lysosomes, developed progressive hypertrophy and underwent cell death. Proteomic and lipidomic analyses revealed accumulation of lysosomal proteins, ceramide biosynthetic enzymes and ceramides. The proteolytic enzyme cathepsin D (CTSD) had impaired maturation. A large proportion of lysosomes were galectin-3 (Lgals3) positive, suggesting cytotoxic lysosomal membrane permeabilization. Collectively, these results demonstrate that iron overload induces lysosomal accumulation and impairs lysosomal function, likely due to iron-induced lipid peroxides that can inhibit lysosomal enzymes.
Keywords: Age-related macular degeneration; Aging; Lysosome; Oxidative stress; Retina.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Ach, T., Huisingh, C., Mcgwin, G., Messinger, J. D., Zhang, T., Bentley, M. J., Gutierrez, D. B., Ablonczy, Z., Smith, R. T., Sloan, K. R.et al. (2014). Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 55, 4832-4841. 10.1167/iovs.14-14802 - DOI - PMC - PubMed
-
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 119, 1417-1436. 10.1001/archopht.119.10.1417 - DOI - PMC - PubMed
-
- Aits, S., Kricker, J., Liu, B., Ellegaard, A.-M., Hämälistö, S., Tvingsholm, S., Corcelle-Termeau, E., Høgh, S., Farkas, T., Holm Jonassen, A.et al. (2015). Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11, 1408-1424. 10.1080/15548627.2015.1063871 - DOI - PMC - PubMed
-
- Alge, C. S., Suppmann, S., Priglinger, S. G., Neubauer, A. S., May, C. A., Hauck, S., Welge-Lussen, U., Ueffing, M. and Kampik, A. (2003). Comparative proteome analysis of native differentiated and cultured dedifferentiated human RPE cells. Invest. Ophthalmol. Vis. Sci. 44, 3629-3641. 10.1167/iovs.02-1225 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

