Autoimmune Atrial Fibrillation
- PMID: 37401487
- PMCID: PMC10399945
- DOI: 10.1161/CIRCULATIONAHA.122.062776
Autoimmune Atrial Fibrillation
Abstract
Background: Atrial fibrillation (AF) is by far the most common cardiac arrhythmia. In about 3% of individuals, AF develops as a primary disorder without any identifiable trigger (idiopathic or historically termed lone AF). In line with the emerging field of autoantibody-related cardiac arrhythmias, the objective of this study was to explore whether autoantibodies targeting cardiac ion channels can underlie unexplained AF.
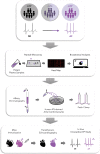
Methods: Peptide microarray was used to screen patient samples for autoantibodies. We compared patients with unexplained AF (n=37 pre-existent AF; n=14 incident AF on follow-up) to age- and sex-matched controls (n=37). Electrophysiological properties of the identified autoantibody were then tested in vitro with the patch clamp technique and in vivo with an experimental mouse model of immunization.
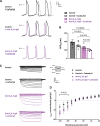
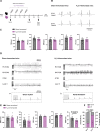
Results: A common autoantibody response against Kir3.4 protein was detected in patients with AF and even before the development of clinically apparent AF. Kir3.4 protein forms a heterotetramer that underlies the cardiac acetylcholine-activated inwardly rectifying K+ current, IKACh. Functional studies on human induced pluripotent stem cell-derived atrial cardiomyocytes showed that anti-Kir3.4 IgG purified from patients with AF shortened action potentials and enhanced the constitutive form of IKACh, both key mediators of AF. To establish a causal relationship, we developed a mouse model of Kir3.4 autoimmunity. Electrophysiological study in Kir3.4-immunized mice showed that Kir3.4 autoantibodies significantly reduced atrial effective refractory period and predisposed animals to a 2.8-fold increased susceptibility to AF.
Conclusions: To our knowledge, this is the first report of an autoimmune pathogenesis of AF with direct evidence of Kir3.4 autoantibody-mediated AF.
Keywords: G protein-coupled inwardly-rectifying potassium channel 4; atrial fibrillation; autoantibody; autoimmunity; inward rectifier potassium channel.
Conflict of interest statement
Figures
Comment in
-
Autoimmune Atrial Fibrillation or Atrial Fibrillation-Induced Autoimmunity? A New Atrial Fibrillation Begets Atrial Fibrillation Pathway?Circulation. 2023 Aug 8;148(6):499-501. doi: 10.1161/CIRCULATIONAHA.123.063672. Epub 2023 Aug 7. Circulation. 2023. PMID: 37549207 No abstract available.
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 - PubMed
-
- Lazzerini PE, Capecchi PL, Laghi-Pasini F, Boutjdir M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat Rev Cardiol. 2017;14:521–535. doi: 10.1038/nrcardio.2017.61 - PubMed

