Extensive remodelling of the cell wall during the development of Staphylococcus aureus bacteraemia
- PMID: 37401629
- PMCID: PMC10328498
- DOI: 10.7554/eLife.87026
Extensive remodelling of the cell wall during the development of Staphylococcus aureus bacteraemia
Abstract
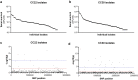
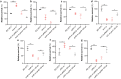
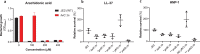
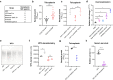
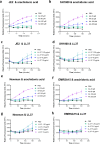
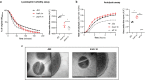
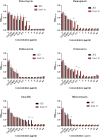
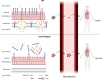
The bloodstream represents a hostile environment that bacteria must overcome to cause bacteraemia. To understand how the major human pathogen Staphylococcus aureus manages this we have utilised a functional genomics approach to identify a number of new loci that affect the ability of the bacteria to survive exposure to serum, the critical first step in the development of bacteraemia. The expression of one of these genes, tcaA, was found to be induced upon exposure to serum, and we show that it is involved in the elaboration of a critical virulence factor, the wall teichoic acids (WTA), within the cell envelope. The activity of the TcaA protein alters the sensitivity of the bacteria to cell wall attacking agents, including antimicrobial peptides, human defence fatty acids, and several antibiotics. This protein also affects the autolytic activity and lysostaphin sensitivity of the bacteria, suggesting that in addition to changing WTA abundance in the cell envelope, it also plays a role in peptidoglycan crosslinking. With TcaA rendering the bacteria more susceptible to serum killing, while simultaneously increasing the abundance of WTA in the cell envelope, it was unclear what effect this protein may have during infection. To explore this, we examined human data and performed murine experimental infections. Collectively, our data suggests that whilst mutations in tcaA are selected for during bacteraemia, this protein positively contributes to the virulence of S. aureus through its involvement in altering the cell wall architecture of the bacteria, a process that appears to play a key role in the development of bacteraemia.
Keywords: Staphylococcus aureus; TcaA; bacteraemia; infectious disease; microbiology; staphylococcus aureus.
Conflict of interest statement
ED, NP, TB, DA, MB, ML, MR, GC, RL, RH, MO, EO, RM, RM No competing interests declared
Figures






Update of
-
Extensive re-modelling of the cell wall during the development of Staphylococcus aureus bacteraemia.bioRxiv [Preprint]. 2023 Apr 24:2023.02.23.529713. doi: 10.1101/2023.02.23.529713. bioRxiv. 2023. Update in: Elife. 2023 Jul 04;12:RP87026. doi: 10.7554/eLife.87026. PMID: 36865143 Free PMC article. Updated. Preprint.
Similar articles
-
Extensive re-modelling of the cell wall during the development of Staphylococcus aureus bacteraemia.bioRxiv [Preprint]. 2023 Apr 24:2023.02.23.529713. doi: 10.1101/2023.02.23.529713. bioRxiv. 2023. Update in: Elife. 2023 Jul 04;12:RP87026. doi: 10.7554/eLife.87026. PMID: 36865143 Free PMC article. Updated. Preprint.
-
GpsB Coordinates Cell Division and Cell Surface Decoration by Wall Teichoic Acids in Staphylococcus aureus.Microbiol Spectr. 2022 Jun 29;10(3):e0141322. doi: 10.1128/spectrum.01413-22. Epub 2022 Jun 1. Microbiol Spectr. 2022. PMID: 35647874 Free PMC article.
-
Wall Teichoic Acids Facilitate the Release of Toxins from the Surface of Staphylococcus aureus.Microbiol Spectr. 2022 Aug 31;10(4):e0101122. doi: 10.1128/spectrum.01011-22. Epub 2022 Jul 7. Microbiol Spectr. 2022. PMID: 35863033 Free PMC article.
-
The staphylococcal surface-glycopolymer wall teichoic acid (WTA) is crucial for complement activation and immunological defense against Staphylococcus aureus infection.Immunobiology. 2016 Oct;221(10):1091-101. doi: 10.1016/j.imbio.2016.06.003. Epub 2016 Jun 15. Immunobiology. 2016. PMID: 27424796 Review.
-
Wall Teichoic Acid in Staphylococcus aureus Host Interaction.Trends Microbiol. 2020 Dec;28(12):985-998. doi: 10.1016/j.tim.2020.05.017. Epub 2020 Jun 12. Trends Microbiol. 2020. PMID: 32540314 Review.
Cited by
-
Host-induced cell wall remodeling impairs opsonophagocytosis of Staphylococcus aureus by neutrophils.mBio. 2024 Aug 14;15(8):e0164324. doi: 10.1128/mbio.01643-24. Epub 2024 Jul 23. mBio. 2024. PMID: 39041819 Free PMC article.
-
Surviving in the blood.Nat Rev Microbiol. 2023 Sep;21(9):553. doi: 10.1038/s41579-023-00951-w. Nat Rev Microbiol. 2023. PMID: 37433956 No abstract available.
-
Anti-staphylococcal fatty acids: mode of action, bacterial resistance and implications for therapeutic application.Microbiology (Reading). 2025 May;171(5):001563. doi: 10.1099/mic.0.001563. Microbiology (Reading). 2025. PMID: 40402078 Free PMC article. Review.
References
-
- Bai AD, Lo CKL, Komorowski AS, Suresh M, Guo K, Garg A, Tandon P, Senecal J, Del Corpo O, Stefanova I, Fogarty C, Butler-Laporte G, McDonald EG, Cheng MP, Morris AM, Loeb M, Lee TC. Staphylococcus aureus bacteraemia mortality: a systematic review and meta-analysis. Clinical Microbiology and Infection. 2022;28:1076–1084. doi: 10.1016/j.cmi.2022.03.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

