State-of-the-art 2023 on gene therapy for phenylketonuria
- PMID: 37401651
- PMCID: PMC10764640
- DOI: 10.1002/jimd.12651
State-of-the-art 2023 on gene therapy for phenylketonuria
Abstract
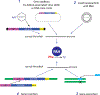
Phenylketonuria (PKU) or hyperphenylalaninemia is considered a paradigm for an inherited (metabolic) liver defect and is, based on murine models that replicate all human pathology, an exemplar model for experimental studies on liver gene therapy. Variants in the PAH gene that lead to hyperphenylalaninemia are never fatal (although devastating if untreated), newborn screening has been available for two generations, and dietary treatment has been considered for a long time as therapeutic and satisfactory. However, significant shortcomings of contemporary dietary treatment of PKU remain. A long list of various gene therapeutic experimental approaches using the classical model for human PKU, the homozygous enu2/2 mouse, witnesses the value of this model to develop treatment for a genetic liver defect. The list of experiments for proof of principle includes recombinant viral (AdV, AAV, and LV) and non-viral (naked DNA or LNP-mRNA) vector delivery methods, combined with gene addition, genome, gene or base editing, and gene insertion or replacement. In addition, a list of current and planned clinical trials for PKU gene therapy is included. This review summarizes, compares, and evaluates the various approaches for the sake of scientific understanding and efficacy testing that may eventually pave the way for safe and efficient human application.
Keywords: adeno-associated virus; gene editing; gene therapy; mRNA therapy; phenylalanine; phenylalanine hydroxylase; phenylketonuria.
© 2023 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

