Sex Hormone-Binding Globulin (SHBG) Maintains Proper Equine Adipose-Derived Stromal Cells (ASCs)' Metabolic Functions and Negatively Regulates their Basal Adipogenic Potential
- PMID: 37402098
- PMCID: PMC10579166
- DOI: 10.1007/s12015-023-10580-8
Sex Hormone-Binding Globulin (SHBG) Maintains Proper Equine Adipose-Derived Stromal Cells (ASCs)' Metabolic Functions and Negatively Regulates their Basal Adipogenic Potential
Abstract
Background: Sex hormone binding globulin (SHBG) deteriorated expression has been recently strongly correlated to increased level of circulating pro-inflammatory cytokines and insulin resistance, which are typical manifestations of equine metabolic syndrome (EMS). Despite previous reports demonstrated the potential therapeutic application of SHBG for liver-related dysfunctions, whether SHBG might modulate equine adipose-derived stem/stromal cells (EqASCs) metabolic machinery remains unknown. Therefore, we evaluated for the first time the impact of SHBG protein on metabolic changes in ASCs isolated from healthy horses.
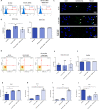
Methods: Beforehand, SHBG protein expression has been experimentally lowered using a predesigned siRNA in EqASCs to verify its metabolic implications and potential therapeutic value. Then, apoptosis profile, oxidative stress, mitochondrial network dynamics and basal adipogenic potential have been evaluated using various molecular and analytical techniques.
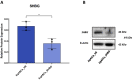
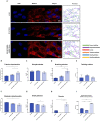
Results: The SHBG knockdown altered the proliferative and metabolic activity of EqASCs, while dampening basal apoptosis via Bax transcript suppression. Furthermore, the cells treated with siRNA were characterized by senescent phenotype, accumulation of reactive oxygen species (ROS), nitric oxide, as well as decreased mitochondrial potential that was shown by mitochondrial membrane depolarization and lower expression of key mitophagy factors: PINK, PARKIN and MFN. The addition of SHBG protein reversed the impaired and senescent phenotype of EMS-like cells that was proven by enhanced proliferative activity, reduced apoptosis resistance, lower ROS accumulation and greater mitochondrial dynamics, which is proposed to be related to a normalization of Bax expression. Crucially, SHBG silencing enhanced the expression of key pro-adipogenic effectors, while decreased the abundance of anti-adipogenic factors namely HIF1-α and FABP4. The addition of exogenous SHBG further depleted the expression of PPARγ and C/EBPα and restored the levels of FABP4 and HIF1-α evoking a strong inhibitory potential toward ASCs adipogenesis.
Conclusion: Herein, we provide for the first time the evidence that SHBG protein in importantly involved in various key metabolic pathways governing EqASCs functions, and more importantly we showed that SHBG negatively affect the basal adipogenic potential of tested ASCs through a FABP4-dependant pathway, and provide thus new insights for the development of potential anti-obesity therapeutic approach in both animals and humans.
Keywords: ASCs; Antiadipogenic; Apoptosis; Knockdown; Mitochondrial dynamics; SHBG.
© 2023. The Author(s).
Conflict of interest statement
Not Applicable.
Figures






References
-
- Saez-Lopez, C., Barbosa-Desongles, A., Hernandez, C., Dyer, R. A., Innis, S. M., Simó, R., & Selva, D. M. (2017). Sex hormone-binding globulin reduction in metabolic disorders may play a role in NAFLD development. EndocrinologyJN.10.1210/en.2016-1668 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

