Clustering of phosphatase RPTPα promotes Src signaling and the arthritogenic action of synovial fibroblasts
- PMID: 37402225
- PMCID: PMC10544828
- DOI: 10.1126/scisignal.abn8668
Clustering of phosphatase RPTPα promotes Src signaling and the arthritogenic action of synovial fibroblasts
Abstract
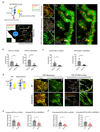
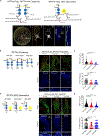
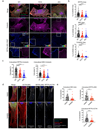
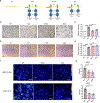
Receptor-type protein phosphatase α (RPTPα) promotes fibroblast-dependent arthritis and fibrosis, in part, by enhancing the activation of the kinase SRC. Synovial fibroblasts lining joint tissue mediate inflammation and tissue damage, and their infiltration into adjacent tissues promotes disease progression. RPTPα includes an ectodomain and two intracellular catalytic domains (D1 and D2) and, in cancer cells, undergoes inhibitory homodimerization, which is dependent on a D1 wedge motif. Through single-molecule localization and labeled molecule interaction microscopy of migrating synovial fibroblasts, we investigated the role of RPTPα dimerization in the activation of SRC, the migration of synovial fibroblasts, and joint damage in a mouse model of arthritis. RPTPα clustered with other RPTPα and with SRC molecules in the context of actin-rich structures. A known dimerization-impairing mutation in the wedge motif (P210L/P211L) and the deletion of the D2 domain reduced RPTPα-RPTPα clustering; however, it also unexpectedly reduced RPTPα-SRC association. The same mutations also reduced recruitment of RPTPα to actin-rich structures and inhibited SRC activation and cellular migration. An antibody against the RPTPα ectodomain that prevented the clustering of RPTPα also inhibited RPTPα-SRC association and SRC activation and attenuated fibroblast migration and joint damage in arthritic mice. A catalytically inactivating RPTPα-C469S mutation protected mice from arthritis and reduced SRC activation in synovial fibroblasts. We conclude that RPTPα clustering retains it to actin-rich structures to promote SRC-mediated fibroblast migration and can be modulated through the extracellular domain.
Figures



References
-
- Alonso A et al., Protein tyrosine phosphatases in the human genome. Cell 117, 699–711 (2004). - PubMed
-
- Lim KL, Lai DS, Kalousek MB, Wang Y, Pallen CJ, Kinetic analysis of two closely related receptor-like protein-tyrosine-phosphatases, PTP alpha and PTP epsilon. Eur J Biochem 245, 693–700 (1997). - PubMed
-
- Blanchetot C, den Hertog J, Multiple interactions between receptor protein-tyrosine phosphatase (RPTP) alpha and membrane-distal protein-tyrosine phosphatase domains of various RPTPs. J Biol Chem 275, 12446–12452 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

