Macrophage-mediated extracellular matrix remodeling controls host Staphylococcus aureus susceptibility in the skin
- PMID: 37402364
- PMCID: PMC10467568
- DOI: 10.1016/j.immuni.2023.06.006
Macrophage-mediated extracellular matrix remodeling controls host Staphylococcus aureus susceptibility in the skin
Abstract
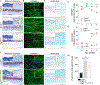
Hypodermis is the predominant site of Staphylococcus aureus infections that cause cellulitis. Given the importance of macrophages in tissue remodeling, we examined the hypodermal macrophages (HDMs) and their impact on host susceptibility to infection. Bulk and single-cell transcriptomics uncovered HDM subsets with CCR2-dichotomy. HDM homeostasis required the fibroblast-derived growth factor CSF1, ablation of which abrogated HDMs from the hypodermal adventitia. Loss of CCR2- HDMs resulted in accumulation of the extracellular matrix component, hyaluronic acid (HA). HDM-mediated HA clearance required sensing by the HA receptor, LYVE-1. Cell-autonomous IGF1 was required for accessibility of AP-1 transcription factor motifs that controlled LYVE-1 expression. Remarkably, loss of HDMs or IGF1 limited Staphylococcus aureus expansion via HA and conferred protection against cellulitis. Our findings reveal a function for macrophages in the regulation of HA with an impact on infection outcomes, which may be harnessed to limit the establishment of infection in the hypodermal niche.
Keywords: Staphylococcus aureus; extracellular matrix; hyaluronic acid; hypodermis; insulin-like growth factor 1; macrophages; skin.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Sundberg JP, Booth CJ, Nanney LB, Fleckman P, and King LE (2017). Comparative Anatomy and Histology (Second Edition) A Mouse, Rat, and Human Atlas (Academic Press; ). 10.1016/C2014-0-03145-0. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

