Time required to initiate a clinical trial in Canada at the onset of the COVID-19 pandemic: an observational research-in-motion study
- PMID: 37402556
- PMCID: PMC10325581
- DOI: 10.9778/cmajo.20220129
Time required to initiate a clinical trial in Canada at the onset of the COVID-19 pandemic: an observational research-in-motion study
Abstract
Background: Randomized controlled trials (RCTs) provide essential evidence to inform practice, but the many necessary steps result in lengthy times to initiation, which is problematic in the case of rapidly emerging infections such as COVID-19. This study aimed to describe the start-up timelines for the Canadian Treatments for COVID-19 (CATCO) RCT.
Methods: We surveyed hospitals participating in CATCO and ethics submission sites using a structured data abstraction form. We measured durations from protocol receipt to site activation and to first patient enrolment, as well as durations of administrative processes, including research ethics board (REB) approval, contract execution and lead times between approvals to site activation.
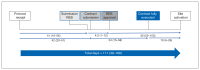
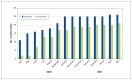
Results: All 48 hospitals (26 academic, 22 community) and 4 ethics submission sites responded. The median time from protocol receipt to trial initiation was 111 days (interquartile range [IQR] 39-189 d, range 15-412 d). The median time between protocol receipt and REB submission was 41 days (IQR 10-56 d, range 4-195 d), from REB submission to approval, 4.5 days (IQR 1-12 d, range 0-169 d), from REB approval to site activation, 35 days (IQR 22-103 d, range 0-169 d), from protocol receipt to contract submission, 42 days (IQR 20-51 d, range 4-237 d), from contract submission to full contract execution, 24 days (IQR 15-58 d, range 5-164 d) and from contract execution to site activation, 10 days (IQR 6-27 d, range 0-216 d). Processes took longer in community hospitals than in academic hospitals.
Interpretation: The time required to initiate RCTs in Canada was lengthy and varied among sites. Adoption of template clinical trial agreements, greater harmonization or central coordination of ethics submissions, and long-term funding of platform trials that engage academic and community hospitals are potential solutions to improve trial start-up efficiency.
© 2023 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests: Jennifer Tsang reports a Physicians’ Services Incorporated Foundation grant to her institution, outside the submitted work. She is cochair of the Canadian Community ICU Research Network (CCIRNet) and vice-chair of the Quest Community Health Centre board of directors. Alexandra Binnie reports a Physicians’ Services Incorporated Foundation grant and a Mohawk Medbuy grant to her institution, outside the submitted work. She is cochair of CCIRNet. Srinivas Murthy reports a grant from the Health Research Foundation, Innovative Medicines Canada, outside the submitted work. No other competing interests were declared.
Figures
References
-
- Simpson CR, Beever D, Challen K, et al. The UK’s pandemic influenza research portfolio: a model for future research on emerging infections. Lancet Infect Dis. 2019;19:e295–300. - PubMed
-
- Ali K, Azher T, Baqi M, et al. Canadian Treatments for COVID-19 (CATCO); Association of Medical Microbiology and Infectious Disease Canada (AMMI) Clinical Research Network and the Canadian Critical Care Trials Group. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194:E242–51. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
